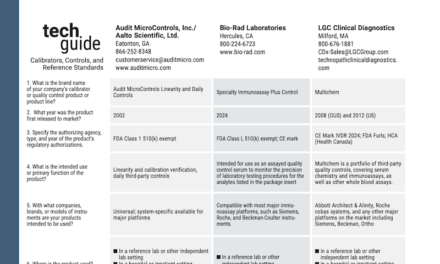
|
In October 2008, Quest Diagnostics Inc, Madison, NJ, initiated a recall/retesting program for what will likely amount to thousands of patients. The problems began in 2007, when the national laboratory changed its testing method for 25-hydroxyvitamin D or Vitamin 25(OH) D testing. The switch to liquid chromatography tandem mass spectrometry (also referred to as LC/MS/MS) resulted in testing errors that went unnoticed by Quest into 2008.
Part of the problem, as revealed to The New York Times (NYT) by Wael A. Salameh, MD, medical director for endocrinology at the Quest Nichols Institute in San Juan Capistrano, Calif, was that “some materials used to calibrate test results had been faulty.”1 In addition, as reported by the NYT, four of Quest’s seven national testing laboratories did not always follow proper procedures, Salameh said.1
The consequence has been thousands of potentially erroneous results—a laboratorian’s worst possible nightmare and one that laboratory processes are often created to avoid. A quality control program is one of those processes and is designed to make sure all of the other elements are working. But what if its own elements are faulty?
“The whole purpose of quality control is to give you confidence in the quality of the results that you are reporting,” says Marcia Zucker, PhD, director of clinical support for Response Biomedical Corp, Vancouver, BC, Canada. The process is often a balancing act. Laboratories want to perform enough testing to ensure quality care at the same time they want to avoid wasting resources on excessive and unnecessary testing.
“Laboratories have to find a way to do what is necessary—not more and not less. But then they have to actually demonstrate that this is the case. It’s a very big challenge to figure out the appropriate metrics to apply in order to develop a robust system,” Zucker says.
Those metrics should include measurements to determine if control materials are actually in control. Regulations often set the minimum requirements, but laboratories are charged with developing quality control procedures that maximize the performance of their specific testing menus and instrumentation. Key elements to a successful program include laboratorian education, appropriate technology and materials, and careful use of checks.
Need to Know It All
Knowledge provides the basis for creating smart testing protocols and is necessary for anyone performing a test and/or running controls, whether it be a laboratorian, nurse, or other clinician. As the laboratorian employee crunch becomes more severe, there is greater reliance on generalists and clinicians, who may not be as familiar with laboratory processes, including quality control, to perform tests. Indeed, even laboratorians may not know as much as they should.
“Many clients have said they don’t know what they are supposed to be doing according to CLIA guidelines,” says John Innocenti, president of Audit MicroControls Inc in Las Vegas. Although medical technology curriculums touch on quality control, advancing technology and changing regulations can outdate knowledge quickly.
Sue Read, manager of QC strategy for Siemens Healthcare Diagnostics, Tarrytown, NY, notes one of the challenges frequently cited during focus groups is investing the time and money to train more technicians on laboratory quality control. Budget and personnel resources tend to be scarce everywhere.
Educational opportunities are not, however. Instruction can range from classes and seminars to simply reading the directions enclosed with the controls. “We get a lot of customers who request new copies of the instructions when they run into problems,” says Innocenti, who suggests those missing copies are often in the trash.
The instructions are important to review because they cover not only proper use but also proper storage. “For example, if a control needs to be stored frozen, the lab really needs to avoid storage in a frost-free freezer because it goes through many defrost cycles, and the control will thaw and freeze over and over again. This can degrade a control,” says Paul Hardy, business unit marketing manager at Bio-Rad Laboratories Quality Systems Division in Irvine, Calif.
In addition to providing specific product information, many manufacturers will also organize or sponsor broader educational opportunities. Associations, such as the American Society for Clinical Pathologists and the American Association for Clinical Chemistry, also produce seminars and conference programs addressing quality control.
These opportunities supplement the knowledge acquired through on-the-job training. But it’s a lot to absorb. According to Greg Cooper, CLS, MHA, manager of clinical standards and practices with Bio-Rad Laboratories, “Labs really need to understand the capability of the testing that occurs in the lab. They need to understand how robust the instrument they are using is. They should be able to characterize how that instrument works in their own unique laboratory environment and with their own staff. Labs also need to understand the capabilities of process control, which can be theoretical and difficult to understand.”
Supplementing Staff with Technology
Technology can help—it can’t replace the laboratorian or a proper quality control program—but it can reduce error, provide guidance, standardize processes, and expand data collection and analysis. Technology can even help with theory.
Theoretical decision-making can be programmed into software and can tell a laboratorian what to do. “For instance, software has been used for many years to calculate certain statistical parameters from quality control data, such as bias, imprecision, and total error for a test. With recent innovations, software can now use these statistical parameters to recommend which quality control rules should be applied. So [laboratorians] don’t have to worry about theoretical understanding or which rules to use,” Cooper says. This is a boon to labs that have a shortage of certified laboratory specialists and rely in part on generalists.
“We’ve seen some really remarkable technological advances in informatics that allow real-time data processing, sharing of information, and proactive monitoring of instruments, ensuring patient results,” Read says.
Those advances have impacted laboratory information systems (which help to standardize processes within a laboratory), middleware, and peer-group analysis programs (which help to standardize outside the lab). “I think that if the technologies continue to incorporate more control processes into a test methodology, that will help laboratories to find that happy medium in their quality control program,” Zucker says.
Comparison with peers is key to that effort. Programs such as these are designed to be invaluable troubleshooting tools. “Users compare the results they are getting to peer groups using the same instrument and controls to analyze for the same analyte,” says Andrew Schaeffer, an R&D scientist with Quantimetrix Corp in Redondo Beach, Calif. With Internet convenience, this can be done as frequently as every day.
When an individual institution’s results stray from the pack, it can raise a red flag. That red flag could be associated with a flaw in the instrument, the controls, or the process. Laboratorians are responsible for ensuring the quality of all of these elements.
Controlling Quality
In general, reputable vendors produce reputable products, including controls. Naturally, compatibility between the instrument and the controls helps, but complete reliance on manufacturer-matched controls is not recommended. Experts suggest laboratories still make proper use of third-party controls.
“Laboratories have a responsibility to track their daily performance with a control and not just read a value off the package insert and say, ‘As long as we get within 20 percent of this number, we’re good,’ ” says Kevin Jones, vice president of sales and marketing for Aalto Scientific Ltd, located in Carlsbad, Calif.
“If we look at how controls have evolved, we can see that the control products produced today—speaking generically—are far superior to the control products offered 20 years ago,” Quantimetrix’s Schaeffer says.
Schaeffer cites characteristics that include improved stability and easier use (if the storage and usage directions are followed). “Typically, most controls have ranges assigned by the manufacturer, and the laboratories don’t have to assign their own if they don’t choose to,” Schaeffer says. This can save a laboratory’s resources, an issue that is of major concern for many.
“Fifteen, 20 years ago, cost was maybe the third or fourth issue when a laboratory looked at controls. Money was important but was not one of the top three priorities. Now, it’s at top of mind,” Innocenti says. Although controls are not the largest line items in a laboratory’s budget, these expenses must still maximize workflow efficiency and drive cost-effectiveness.
There are a number of ways laboratories can increase the value of their control purchases while reducing cost:
- One is to use a multiconstituent control, one control material that can run many assays. Rather than buy four, five, or six controls, a laboratory can buy one, more useful, control. According to Innocenti, this can save up to $1,000.
- The industry has trended to human-based controls, which tend to behave more similarly to patient samples, although there are some animal serum-based controls still on the market.
- A control should have a very long shelf life and open-vial stability, which reduce waste and the frequency of crossover studies.
- Controls should also be purchased in lots that minimize the need for crossover studies while maximizing shelf life.
The debate surrounding lyophilized versus liquid controls continues. Some argue that lyophilized products offer greater value through longer shelf lives, greater stability, and multianalyte use. Others believe liquid controls offer advantages related to savings in laboratorian time and a reduction in the risk of human error introduced by manual mixing.
 |
| Liquid controls can help to reduce manual labor, saving time and reducing risk associated with human error. |
Laboratories have also looked to equivalent quality control (EQC) to help reduce expense by reducing the frequency of testing quality control materials. However, some experts caution the cost may not be worth it. “EQC basically allows a laboratory to reduce the frequency of quality control based on certain conditions or circumstances occurring as defined in the interpretive guidelines for CLIA,” Bio-Rad Laboratories’ Cooper summarizes.
But, Cooper continues, this may not be enough. Laboratories shouldn’t just rely on meeting the minimum requirements. Rather, they should continually evaluate their quality control programs for areas of improvement. “If they really investigate device performance and laboratory conditions that might contribute to risk, I think they will find they need to run quality control a bit more frequently for some tests than what may be allowed or required by regulation,” Cooper says.
Control in the Future
Currently, the Clinical and Laboratory Standards Institute (CLSI) has two subcommittees working on documents regarding quality control. CLSI EP22, Presentation of Manufacturer Risk Mitigation Information for Users of In Vitro Diagnostic Devices, focuses on the information that a manufacturer should provide to users about device risk mitigation features. The purpose is to help laboratories make appropriate and effective decisions about the quality control testing that is needed.
CLSI EP23, Laboratory Quality Control Plan Based on Risk Management, is intended to guide laboratories in the development of a quality plan. It suggests that alternative control processes should take shape around the relevant risk factors—including those defined in the manufacturer device information and those present in the laboratory’s unique environment—which could contribute to reporting test results that, if acted upon, could result in harm to the patient.
 To monitor developments in quality controls, bookmark our website. |
These documents will help standardize processes across laboratories, leading to greater consistency in results across institutions and, ideally, disciplines. As new tests are developed, new controls and processes will be developed as well. Some of the more promising opportunities lie in the fields of molecular diagnostics, nanotechnology, and personalized medicine (eg, drug monitoring).
Where gaps exist, laboratories fill in—even new tests require controls to ensure quality results and avoid negative consequences in patient care. The goal is to keep the process as simple, error-proof, and inexpensive as possible while ensuring success—and avoiding having to retest thousands of patients.
Renee Diiulio is a contributing writer for CLP.
Reference
- Pollack A. Quest acknowledges errors in vitamin D tests. The New York Times. January 8, 2009. Available at: www.nytimes.com/2009/01/08/business/08labtest.html?_r=1&scp=1&sq=vitamin%20D%20quest&st=cse. Accessed February 6, 2009.