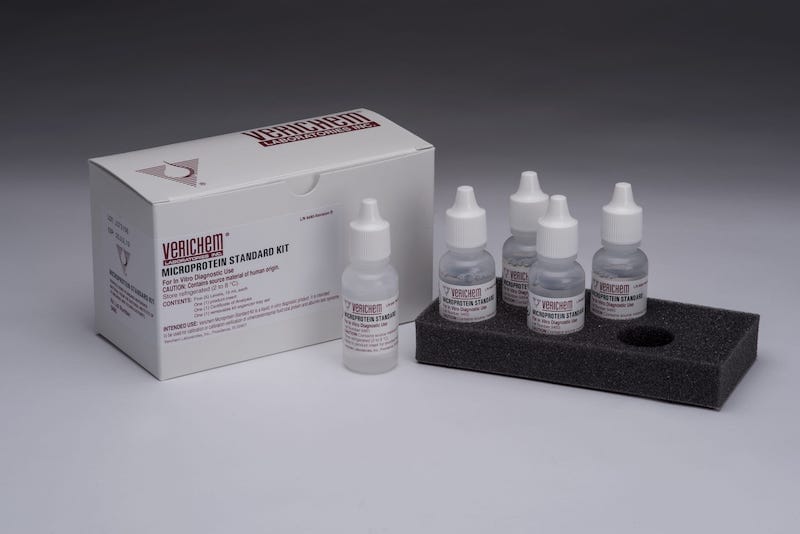
Liquid stable and ready-to-use clinical reference materials for microprotein testing are now available from Verichem Laboratories, which are intended for the calibration and calibration verification of clinical systems testing for total protein and albumin in urine and cerebral spinal fluid (CSF) samples.
Accurate measurement of microprotein and microalbumin levels in urine are useful in the early detection of kidney damage, while testing CSF is useful in uncovering abnormalities within the central nervous system. As such, the availability of these materials from Verichem Laboratories is aimed to address the needs of a wide variety of laboratory professionals, including those involved with routine clinical testing, medical research applications, and in the development and manufacturing of IVD products.
Suitable for use with turbidimetric and colorimetric test methodologies, Verichem’s Clinical Reference Materials for Microprotein incorporates human protein components for optimum reactivity. The five level set of materials provides 10 individual certified component concentrations in total, with each of the certified gravimetric concentration levels employing a distinctive set point design with equidistant target concentrations to address the current CLIA requirements for clinical system accuracy, sensitivity, linearity, and reportable range.
The materials are packaged as a kit, with fifteen milliliters of material at each of the five levels, which provides ample volumes sufficient enough to cover multiple calibration verification events, any unexpected system trouble-shooting, or any additional testing procedures.
The materials are filled in translucent polyethylene dropper vials and offer an outstanding shelf-life stability claim of 24 months from date of manufacture. Plus, a Certificate of Analysis is included with each kit indicating traceability to available NIST and/or ERM Standard Reference Materials.