What can the laboratory community do to solve the problem?
By Gary Tufel
In recent years, the long-smoldering problem of hospital-acquired infections (HAIs) has received increasing attention from healthcare authorities in the United States as well as internationally. But with the dramatic increase in antibiotic-resistant organisms, many health professionals now believe that the need for treating and preventing HAIs has reached a crisis stage. Clinical labs are working to meet the challenges of their roles in diagnosing and monitoring HAIs, but a variety of factors make those tasks very difficult.
One difficulty involves the wide variety of settings affected by the crisis. Recognizing that the challenge of detecting and preventing HAIs is not limited to hospitals alone, the US Centers for Disease Control and Prevention (CDC) now defines HAIs as “healthcare-associated infections.” (For more information, see the sidebar “New Terms for Growing Complexity.”)
Another set of difficulties relates to the sheer number of patients affected by HAIs. In March 2014, CDC researchers published the results of a multiyear effort to define the magnitude of the HAI crisis.1 Based on a prevalence survey conducted in 2011 among a large sample of US acute care hospitals, CDC’s report provides an updated national estimate of the overall problem of HAIs.

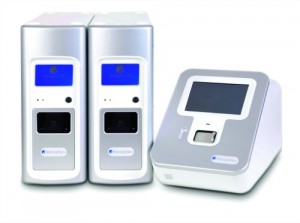
The Accelerate ID/AST system uses automated microscopy and time-lapse imaging to provide infectious organism identification and antibiotic susceptibility results in hours instead of days.
The CDC survey found that on any given day, about 1 in 25 hospital patients has at least one healthcare-associated infection. In 2011, there were an estimated 722,000 HAIs in US acute care hospitals. About 75,000 hospital patients with HAIs died during their hospitalizations. And more than half of all HAIs occurred outside of the intensive care unit (ICU; Table 1).
The difficulties of the HAI crisis also raise serious legal and financial issues for both patients and providers. Considering the major sources that contribute to the rise of HAIs, patients and their attorneys constitute a standing source of potential lawsuits. Meanwhile, the Centers for Medicare & Medicaid Services (CMS) have cracked down on reimbursements for care necessitated by HAIs, effectively forcing providers to cover the costs on their own. Clinical laboratories can hardly expect to remain unaffected by such issues, even as they go about their principal tasks of diagnosis and monitoring.
LAB CHALLENGES
Clinical labs are a vital link in the process of preventing, diagnosing, and treating HAIs. Their function—using blood, urine, and other patient samples to diagnose HAIs and drug-resistant infections—is essential for determining which antibiotics will be most effective in treating each case.
Labs face two major challenges while performing this function, says Joen Johansen, head of marketing for Accelerate Diagnostics, Tucson, Ariz. The first challenge is the time required to identify causative pathogens and determine their antibiotic susceptibility profiles. Existing methods can take days, he says, and the longer a patient receives ineffective therapy for an infection, the lower the chance of survival (Table 2).
The second challenge is financial: that is, the diminishing rates of reimbursement for hospitals, which in turn directly affect lab revenues. Medicare inpatient reimbursement rates are established in accordance with CMS’s diagnosis-related grouping system, which classifies hospital cases into one of about 500 groups, each incorporating all the pertinent products and services provided for the group by the hospital, from lab tests to appendectomies.
In the case of HAIs, says Johansen, laboratories are also charged to determine whether patient infections represent preventable conditions—and therefore cases not eligible for reimbursement—according to recently implemented Medicare rules.
“It’s a dilemma for hospitals, which are facing lower reimbursement rates but higher costs because of longer patient stays due to the infections,” says Johansen. “Even private insurers often follow Medicare when setting reimbursement rates.”
Moreover, he says, an aging population will require increased access to healthcare services, so the problem will keep growing. Patients with pre-existing or newly diagnosed conditions may have a heightened risk for infection, raising new concerns about how to manage their care. (For more information, see the sidebar, “Blood Glucose and Infections.”) And on top of the problems already being encountered in acute care hospitals, additional concerns may arise in the case of alternative care facilities that have less rigorous means of addressing HAIs.
So the challenge for labs, says Johansen, has become how to diagnose infections rapidly, in order to help clinicians optimize treatment as early as possible, thereby speeding the recovery of those with HAIs and reducing their time in the hospital.
BRIGHT SPOTS
Kathy Warye, worldwide vice president for infection prevention at Becton Dickinson and Co (BD), Franklin Lakes, NJ, agrees that HAIs are a problem that has reached crisis stage. Nevertheless, she says, there are some bright spots among providers’ efforts. A CDC report of March 2014 indicated that from 2008 through 2012, hospitals reduced central line-associated bloodstream infections by 44% and surgical-site infections by 20%.2 In addition, methicillin-resistant Staphylococcus aureus (MRSA) infections dropped by 4%.
Warye says one reason for the improved MRSA statistics is that hospitals are able to identify colonized and infected patients more rapidly and take appropriate action to prevent infection and transmission. Hospitals that are combining screening with comprehensive infection prevention and control strategies have seen positive results. Culture change programs, which stress compliance with infection control measures, have also been instrumental in driving reduction in HAIs.
“The advent of the HAI ‘bundle’—which stresses consistent compliance with a small number of critical, evidence-based infection control measures—is enabling an increasing number of institutions to achieve and sustain significant reductions in catheter-related bloodstream infections (CRBSIs) and ventilator-associated pneumonia,” says Warye. “Utilization of this approach, mostly done in the ICU, is now being expanded outside the ICU as well.”
However, not everything in the CDC report of March 2014 was rosy. While C. difficile infections declined 2% from 2011 to 2012, this multidrug-resistant organism remains problematic for many institutions. Meanwhile, catheter-associated urinary tract infections rose 3% from 2009 to 2012.
ANTIBIOTIC RESISTANCE
With so much apparent progress, why so much concern?
High on the list of worries is the fact that an increasing number of organisms have become resistant to antibiotics as a result of overprescribing and misuse of the drugs. “We are seeing organisms for which there are no effective therapies,” says Warye. “This situation, in combination with the currently very weak pipeline for the development of new antibiotics, threatens to take us back to pre-antibiotic days,” she says.
Given the increasing number of untreatable infections and the long development timeline for new drugs—typically, 20 years from initial R&D to regulatory approval—activities to deal with HAIs in the foreseeable future will focus on prevention, not treatment or control. That’s why a close partnership between clinical labs and infection prevention specialists is so vital.
In September 2013, CDC published a first-ever report on the threat of antibiotic resistance in the United States.3 The report estimates that 2 million people in the US contract antibiotic-resistant infections every year, and more than 20,000 people die as a result. The economic burden for managing these infections exceeds $20 billion per year in direct healthcare costs.
“The high mortality and significant costs associated with these infections are due, in large part, to a fundamental limitation based on hospital adoption of rapid diagnostics,” says Michael McGarrity, president and CEO of Nanosphere Inc, a molecular diagnostics company based in Northbrook, Ill. “In hospitals where rapid molecular testing methods have not been adopted, healthcare providers often do not know what kind of infection they are treating until conventional laboratory results are delivered—sometimes 2 to 3 days after their initial assessment.
Infections caused by antibiotic-resistant bacteria, such as carbapenem-resistant Enterobacteriaceae, MRSA, and vancomycin-resistant Enterococcus (VRE)—three of the most common and deadly HAIs—pose a particularly challenging threat to effective disease management, McGarrity adds.
“Technologies that enable rapid identification of infectious pathogens and drug-resistance markers, on the other hand, can help inform targeted patient treatment decisions earlier and reduce overuse of broad-spectrum empiric antibiotics,” says McGarrity. “This is critical, as the number of new antibacterial agents has fallen by 88% over the past 3 decades, leaving few last-line therapies that are capable of killing these antibiotic-resistant superbugs.”4
The problem of antibiotic resistance is serious and enormous, extending its reach to every corner of the globe. The World Economic Forum’s Global Risks 2013 report asserted that antibiotic resistance endangers not only the world’s health systems but also its social and economic stability.5 And the organization’s Global Risks 2014 report included antibiotic resistance among the top risks confronting the world, citing the danger of losing control over deadly diseases due to the growing resistance of deadly bacteria to known antibiotics.6
Adding to the layers of concern among healthcare professionals, an April 2014 report by the World Health Organization termed the problem of antibiotic resistance so serious that it threatens the achievements of modern medicine. “A post-antibiotic era—in which common infections and minor injuries can kill—far from being an apocalyptic fantasy, is instead a very real possibility for the 21st century.”7
With such aligned concern throughout the health policy community, government action could not be far behind. At the beginning of July, British Prime Minister David Cameron commissioned an independent review of the economic issues surrounding antimicrobial resistance, with the goal of encouraging and accelerating the discovery and development of new generations of antibiotics.8
“Resistance to antibiotics is now a very real and worrying threat, as bacteria mutate to become immune to their effects,” said Cameron. Noting that 25,000 people in Europe die each year from infections resistant to antibiotic drugs, he added, “This is not some distant threat but something happening right now.
”If we fail to act, we are looking at an almost unthinkable scenario where antibiotics no longer work and we are cast back into the dark ages of medicine where treatable infections and injuries will kill once again.”
Efforts to halt the decline of effective antibiotics will also require the commitment and involvement of professionals in the field. “The use of appropriate diagnostic tools to identify infections and ensure that treatment is pathogen-directed will be vital for patient outcomes as well as for our ability to preserve critical antibiotics,” says Warye. She cites the efforts of the Association for Professionals in Infection Control and Epidemiology and the American Society for Microbiology for keeping the issues at the forefront, and providing education and opportunities for lab and infection prevention professionals to forge strong partnerships to address the problem (For more information, see the sidebar, “Prevention Through Immunization.”)
And prevention is evolving, she says. Currently, about half of US hospitals have antibiotic stewardship programs, which aim to improve the use of antibiotics by curbing overuse and misuse (more than 50% of antibiotics are misprescribed). “We need to ensure the judicious use of antibiotics, first by stopping the prescribing of antibiotics for conditions that they cannot treat and then to ensure that broad-spectrum antibiotics are used only when indicated,” says Warye.
“Targeted, pathogen-directed therapy—the best bug-drug match—leads to better outcomes, helps to preserve the broad-spectrum antibiotics that are under pressure, and reduces costs related to antibiotic use.”
CUTTING COSTS
Reducing the incidence of HAIs will bring health benefits to patients, says Warye, but it’s also imperative in order to eliminate the significant avoidable costs they generate—an increasing portion of which will no longer be reimbursed by CMS. CRBSIs, for instance, are among the preventable healthcare-associated conditions that are no longer reimbursed by CMS.
Savings to the healthcare system from reducing the incidence of even a single HAI can be substantial. Noting that CRBSIs “are frequently observed in the intensive care unit (ICU) and are a serious cause of morbidity and mortality in the United States,” a 2011 review set out to summarize current information about the costs of the infection in ICU settings.9 At the time of the article, the cost of a single CRBSI incident was between $33,000 and $44,000 in the general adult ICU, between $54,000 and $75,000 in the adult surgical ICU, and approximately $49,000 in the pediatric ICU.
However, the review also found that CRBSIs are associated with reimbursement that is more than $26,000 less than costs. “Hospital and clinical decision-makers should be aware of the high cost of CRBSIs in the ICU, the relatively poor reimbursement, and the implied high value of prevention efforts.”
“The good news,” concluded the report, “is that there are methods for prevention of CRBSIs that are available and are likely to be cost-effective given the stylized facts about costs and reimbursement reported here.”
BUILDING BETTER TESTING

BD’s Kiestra total lab automation (TLA) solution supports antibiotic stewardship efforts by speeding the flow of critical information.
Taken together, says Warye, all of these factors should be driving the development of more and better diagnostics for the clinical lab. Rapid diagnostics that can help stop HAIs before they start, and antimicrobial susceptibility testing to ensure that therapies are targeted to the organism, will both continue to be vital tools in the battle to prevent HAIs and preserve the efficacy of critical antibiotics.
Nanosphere’s McGarrity agrees that the best way to combat HAIs is to administer targeted antimicrobial therapy to ensure that the right drug goes to the right patient, based on identification of the causative bacteria and antimicrobial resistance. “By implementing technologies that can deliver rapid and accurate diagnostic results to clinicians within a window of opportunity when treatment decisions are being made, clinical labs can have a dramatic impact on not only patient outcomes, but also on the effectiveness of hospital antimicrobial stewardship teams, length of stay, and healthcare economics,” he says.
As labs gain a greater awareness of the HAI crisis, they should also understand the importance of their role in helping patients get treated faster, says Johansen. In order to fulfill that role, individual labs need access to new technologies that can provide better information faster.
“The 2 to 3 days now required for a lab to turn around a suspected HAI sample and provide antibiotic information is too long,” he says. “In the meantime, the patient’s condition might actually get worse as a result of being treated with the wrong antibiotic. We need to identify the organisms faster and quickly determine which antibiotics work most effectively for the infection.
“A new Accelerate product that uses automated microscopy and time-lapse imaging to watch how organisms respond to antibiotics can reduce turnaround time for organism identification (ID) and antibiotic susceptibility (AST) results to just hours instead of days,” says Johansen. “This enables the lab to support clinicians in making decisions based not on empirical guesses but on hard facts—and that can make a huge difference in determining the right antibiotics to use.”
The Accelerate ID/AST system is a fully automated, modular system that can be scaled to fit any lab. System setup takes only 2 minutes, making it possible to provide test results when labs, clinicians, and patients need them most. According to Johansen, the system is expected to launch in Europe in early 2015 and in the United States in early 2016.
Nanosphere’s McGarrity agrees that organism identification and antibiotic susceptibility are two key points of information necessary for ensuring that patients receive the correct treatment as quickly as possible. He adds that highly targeted and specific multiplex molecular diagnostic tests, such as the Verigene system by Nanosphere, can rapidly and accurately identify both the infection-causing bacteria and resistance to commonly used antibiotics within 2.5 hours of blood culture positivity.
“The Verigene gram-positive blood culture test (BC-GP) and Verigene gram-negative blood culture test (BC-GN) respectively identify genus, species, and genetic resistance determinants for a broad panel of gram-positive and gram-negative bacteria directly from positive blood culture bottles up to 48 hours faster than conventional methods,” he says.
For providers and their patients, the application of such advanced diagnostic systems means that treatment can be initiated much earlier, says McGarrity. “A recent study demonstrated that following implementation of Verigene BC-GP, there was a 26-hour reduction in the time from sample collection to the first dose of appropriate antibiotics for patients with methicillin-sensitive Staphylococcus aureus and VRE infections.”10

The BD Max automated molecular platform is capable of running both FDA-cleared and open-system assays
But the conditions we are facing, Warye says, are going to challenge us to think beyond the developments of the past several years. In addition to speed, for instance, flexibility will also be important where new and emerging organisms are concerned. The BD Max automated molecular platform is capable of running both FDA-cleared and open-system assays.
Lab automation, by increasing overall efficiency and speeding the flow of critical information, will also advance the judicious use of antibiotics, she adds. BD’s Kiestra total lab automation solution, which combines capacity and flexibility with connectivity and integration, will also support stewardship efforts and reduction of HAIs.
“A holistic approach that combines automation, standardization, flexibility, and connectivity will go furthest in enabling labs to address the challenge of HAIs and emerging organisms,” says Warye.
CONCLUSION
Test developers and laboratorians are already working hard to reduce the threat of HAIs to patients and populations throughout the world. But with the number and variety of antibiotic-resistant organisms still on the rise, future success will require focused and coordinated efforts from an even broader range of healthcare professionals. As ever, clinical laboratorians will have a central role to play in identifying target organisms, defining optimal therapies, and ultimately protecting the past and future achievements of modern medicine.
Gary Tufel is a contributing writer for CLP. For further information, contact CLP chief editor Steve Halasey via [email protected].
REFERENCES
1. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of healthcare-associated infections. N Engl J Med. 2014;370:1198–1208; doi: 10.1056/NEJMoa1306801.
2. National and state healthcare-associated infections progress report. Atlanta: Centers for Disease Control and Prevention, 2014. Available at: www.cdc.gov/HAI/pdfs/progress-report/hai-progress-report.pdf. Accessed August 13, 2014.
3. Antibiotic Resistance Threats in the United States, 2013. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/index.html. Accessed July 14, 2014.
4. Boucher HW, Talbot GH, Benjamin DK Jr, et al. 10 × ‘20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(12):1685–1694; Epub April 17, 2013; doi: 10.1093/cid/cit152.
5. Global risks 2013. Geneva: World Economic Forum, 2013. Available at: http://reports.weforum.org/global-risks-2013/. Accessed August 14, 2014.
6. Global risks 2014. Geneva: World Economic Forum, 2014. Available at: http://reports.weforum.org/global-risks-2014/. Accessed August 14, 2014.
7. Antimicrobial resistance: global report on surveillance. Geneva: World Health Organization, 2014. Available at: www.who.int/drugresistance/documents/surveillancereport/en/. Accessed August 14, 2014.
8. Prime minister warns of global threat of antibiotic resistance. London: UK Department of Health, 2014. Available at: https://www.gov.uk/government/news/prime-minister-warns-of-global-threat-of-antibiotic-resistance. Accessed August 14, 2104.
9. Hollenbeak CS. The cost of catheter-related bloodstream infections: implications for the value of prevention. J Infus Nurs. 2011;34(5):309–313; doi: 10.1097/NAN.0b013e3182285e43.
10. Beal SG, Thomas C, Dhiman N, et al. De-escalation of antibiotics in response to the Nanosphere Verigene gram-positive blood culture assay. Poster presentation C-1141 to the annual meeting of the American Society for Microbiology, Boston, May 17–20, 2014; abstract available at: www.asmonlineeducation.com/php/asm2014abstracts/data/papers/C-1141.htm.