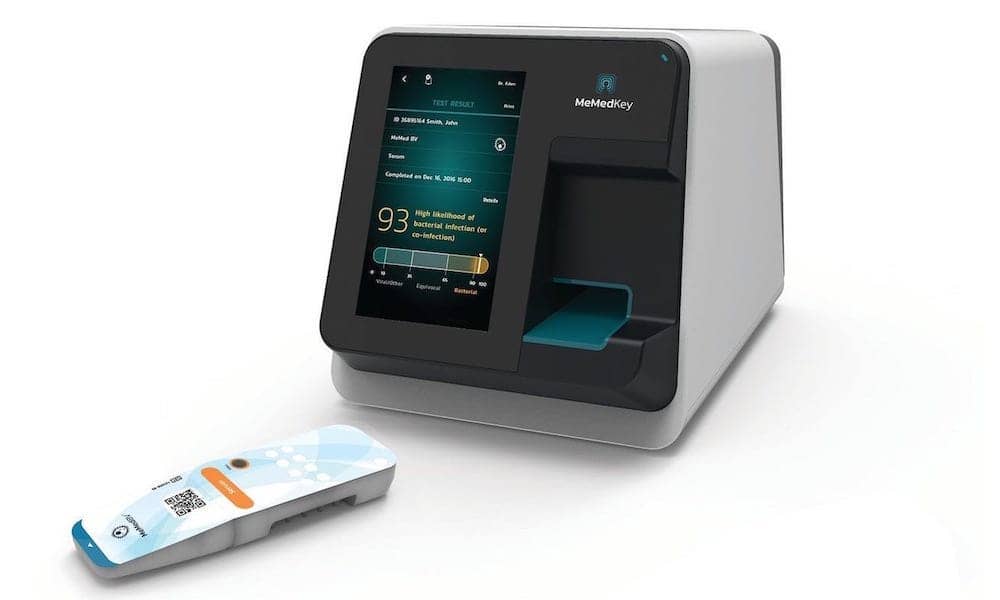
The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for technology to help healthcare providers distinguish between bacterial and viral infections in both children and adults. Clearance was granted for the use of the MeMed BV test on the point-of-need platform MeMed Key, both developed by MeMed, Haifa, Israel, a company specializing in advanced host-response technologies.
MeMed BV measures and computationally integrates the levels of three immune system proteins: TRAIL, IP-10, and CRP. When run on the MeMed Key platform, MeMed BV provides a result within 15 minutes.
“Host-response technologies are a new frontier in the management of patients with infectious diseases, with great potential to improve patient outcomes,” says Sergey Motov, MD, professor of emergency medicine at Maimonides Medical Center, New York. “Every day, I see adults with a complicated medical history presenting to the emergency room with a suspected respiratory tract infection. A technology like MeMed BV can significantly aid in their management.”
Bacterial and viral infections are often clinically indistinguishable, leading to the prescription of antibiotics for the treatment of viral infections, for which antibiotics are ineffective. Antibiotic misuse drives the emergence of antimicrobial resistance, one of today’s biggest healthcare challenges.
“For those of us who care for acutely ill children, we have been waiting decades for accurate, rapid diagnostics to confidently guide the care of moderately ill children without a clear focus of infection or recognizable viral illness,” says Rich Bachur, MD, professor of pediatrics and emergency medicine at Harvard Medical School and chief of the division of emergency medicine for Boston Children’s Hospital. “This novel test offers promise to help differentiate those children with self-limited viral illness from those with possible bacterial infection, thereby supporting the judicious use of antibiotics.”
MeMed’s technology decodes the body’s immune response to infection (the host response) rather than focusing on detecting the presence of a microbe. According to the company, this allows robust diagnosis when the infection site is inaccessible or unknown, even when the pathogen is undetectable using conventional tests, or when the cause of infection is an emerging new pathogen. It also enables better informed antibiotic treatment decisions, an essential tool in the fight against resistant bacteria.
“We are now using MeMed BV in my department routinely to aid in determining whether a child with fever has a bacterial or viral infection,” says Adi Klein, MD, director of the pediatric division for Hillel Yaffe Medical Center, Hadera, Israel, and the head of the Israeli Clinical Pediatric Society. “For example, we recently had a complicated case of a young child with fever but without a clear source. MeMed BV helped in early identification of a severe bacterial infection that would otherwise be masked by viral PCR detection, lead to a change in the course of treatment, and made a big difference in the patient’s outcome. Introducing MeMed’s technology has had a significant impact on our medical practice, enabling us to be better stewards of antibiotics and improving patient outcomes.”
FDA clearance was based on a multi-center blinded clinical validation study that enrolled more than one thousand children and adults and addressed goals laid out in the U.S. National Action Plan for Combating Antibiotic Resistant Bacteria. According to the company, the test provides highly accurate results with area under the curve of 90 percent and 97 percent (primary and secondary endpoints). MeMed has established its U.S. base in Boston and is ramping up commercial activities to ensure broad availability of its products across the United States.
“It has been a decade-long journey to reach this point from concept to impacting patient lives,” says Eran Eden, PhD, cofounder and CEO of MeMed. “This FDA clearance is a breakthrough moment in the field of advanced host-response and could not have been achieved without the dedication of the MeMed team, our clinical partners in the United States and around the globe, and the support of the U.S. Department of Defense and EU Commission.”
For more information, visit MeMed.
Feature Image: When run on the MeMed Key platform, the MeMed BV test decodes the immune response to accurately distinguish between bacterial or viral infections within minutes. Photo: MeMed