Summary:
Amsbio, in collaboration with Nordmark Biochemicals, has launched GMP-grade Collagenase and Neutral Protease NB enzymes to support high-purity, regulatory-compliant tissue dissociation workflows for both research and clinical applications.
Takeaways:
- The enzymes, sourced from Clostridium histolyticum, are essential for isolating viable cells in regenerative medicine and stem cell therapies.
- GMP-grade production ensures compliance with EU, FDA, and ISO standards, with strict quality control for safety, consistency, and high cell yield.
- Amsbio’s translational model allows seamless transition from research to clinical use by providing research and GMP enzyme formats with equivalent activity.
Amsbio, in association with Nordmark Biochemicals, has expanded its range of Collagenase and Neutral Protease NB enzymes with GMP grade formats and protocols to support advanced tissue dissociation workflows for applications from research to clinical use.
Enzymes Are Derived from Clostridium Histolyticum
These enzymes, derived from Clostridium histolyticum, are critical raw materials for isolating viable cells from complex tissues, an essential step in tissue engineering, cell therapy and regenerative medicine. Their clinical relevance is reinforced by recent studies to set up a protocol for isolation, manufacturing, and storage of mesenchymal stem cells (MSCs) under GMP-compliance (Williams et al. 2025*).
To meet the demanding purity requirements for these applications, production facilities for these Collagenase and Neutral Protease enzymes are certified as Good Manufacturing Practice (GMP) compliant, following EU Guidelines for Good Manufacturing Practice as well as other national and international regulatory frameworks such as the Pharmacopoeias, DIN EN ISO Standards and guidelines of the US Food and Drug Administration (FDA), CFR and many more.
The superb quality and performance of these new GMP-grade products is guaranteed by stringent quality control ensuring reliable lot-to-lot consistency and delivering high yields of viable cells with every use. TSE (Transmissible Spongiform Encephalopathy) safety of these products is clinically certified and Virus validation studies performed.
Amsbio Offers a Translational Collagenese Model
Amsbio offers a “Translational Collagenase model” where research grade products are offered alongside GMP alternatives of equivalent activity. The end-user can optimize their protocols with the research grade, then later easily switch to the GMP version for the clinical environment without making any changes to the protocol.
*Williams et al – BMC Molecular and Cell Biology, 2025, 26(1), 1-10
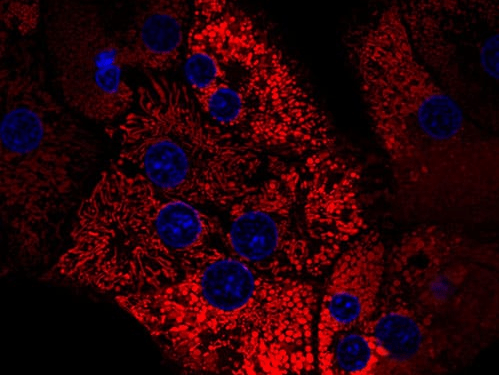
Featured Image: Rat hepatocytes isolated using Collagenase NB 4G (Proved Grade): mitochondria stained with TMRM (red), nuclei stained with Hoechst 33342 (blue). Image: Dr. Pless, University Essen (Germany)