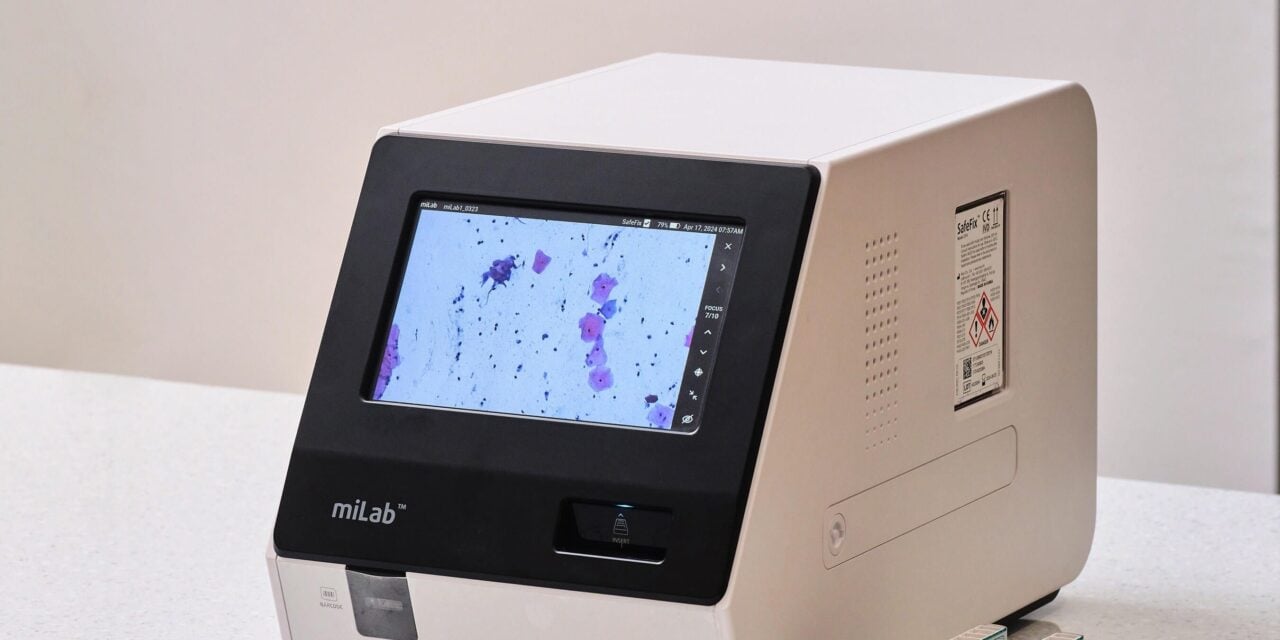
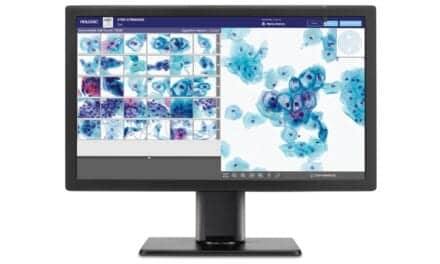
The system automates cervical cytology with AI-powered sample preparation, imaging, and analysis capabilities.
Noul Co Ltd has secured US Food and Drug Administration (FDA) registration for its cervical cancer diagnostic cartridge miLab Cartridge CER and clearing agent SafeFix CER, core components of the company’s cervical cancer diagnostic solution miLab CER.
The registration fulfills minimum regulatory requirements for US market entry. Beginning in October, the South Korea-based medical AI company will launch commercial shipments of miLab CER in Europe and Latin America, starting with countries where regulatory clearance has been obtained.
The FDA device listing provides Noul a foothold to enter the US market with its cervical cancer portfolio, adding to its existing miLab platform for malaria diagnostics and blood analysis. The company is preparing phased FDA 510(k) submissions for disease-specific analysis software to be integrated into the miLab platform.
“The registration of our miLab CER product line marks an important milestone for entering the US market and a catalyst for our global expansion,” says David Lim, CEO, in a release. “In parallel with the upcoming launches in Europe and Latin America, shipments will begin this month to countries including Qatar, Panama, and the UK, where regulatory approvals are already in place.”
Addressing Screening Disparities
Cervical cancer survival rates highlight the critical need for early detection. In the US, the five-year survival rate exceeds 90% when diagnosed early but drops below 20% at late stages. Screening disparities remain significant, with uninsured and low-income women participating at rates more than 20% lower than average, and African American women facing a 60% higher incidence and more than double the mortality rate compared to white women.
Noul’s miLab CER automates cervical cytology with an integrated AI-powered system that performs sample preparation, imaging, and analysis. The product was named one of three recommended technologies for cervical cancer triage alongside Roche and Hologic in the 2024 WHO-Unitaid report.
Earlier this year, Noul secured global supply agreements in six Latin American countries, including Panama, as well as Qatar, prior to launch. The company has since obtained registration in Switzerland and regulatory clearance in Vietnam.
Platform Capabilities
The miLab Platform fully automates the microscopic diagnostic process from sample preparation to AI-based image analysis, delivering lab-grade blood and cancer diagnostics at the point of care. Within 15 minutes, miLab produces results even in low-resource settings.
In 2022, miLab was described in a Unitaid report as “the most advanced digital microscope and fully integrated bench-top platform” for malaria diagnostics. The system is now deployed in 28 countries, used by pharmaceutical companies, hospitals, labs, and public health institutions worldwide.
Founded in December 2015, Noul trades on the Korea Exchange under ticker 376930.KQ. The company specializes in blood and cancer diagnostics through convergence of AI, biotechnology, and compact robotics.
Photo caption: Noul’s miLab platform with cervical cancer diagnostic cartridges (miLab Cartridge CER)
Photo credit: Noul