At the end of November 2017, FDA issued two draft guidance documents directed primarily toward manufacturers seeking waived complexity status for their commercially available in vitro diagnostics (IVDs) under the terms of the Clinical Laboratory Improvement Amendments of 1988 (CLIA).
The first guidance, Select Updates for Recommendations for CLIA Waiver Applications for Manufacturers of In Vitro Diagnostic Devices, represents a required update to FDA’s previous recommendations for demonstrating that a test is accurate and carries insignificant risk of an erroneous result.1 The new guidance will put forward the agency’s current thinking about the appropriate use of comparable performance between a user in a CLIA-waived facility and a user in a moderate complexity laboratory to demonstrate the accuracy of a test.
The second guidance, Recommendations for Dual 510(k) and CLIA Waiver by Application Studies, describes the agency’s expectations regarding study designs for generating data that support both premarket notification (510(k)) clearance and CLIA waiver by application.2 FDA believes that increased use of this pathway will speed the process of bringing simple and accurate IVD devices to CLIA-waived settings, which will better serve patients and providers.
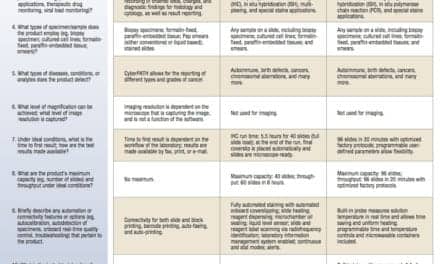
Each of these guidance documents focuses on identifying the conditions and methods of testing appropriate for ensuring that an IVD intended for broad commercial availability and use in CLIA-waived settings will produce accurate results when used correctly by untrained operators. The agency recommends, for instance, that applicants evaluate test performance “in settings designed to replicate, as closely as possible, the actual CLIA-waived settings and patients/samples.”1 The agency describes in detail the number and type of testing sites, operators, and patient samples that should be included in the manufacturer’s study designs. And in the guidance on the dual submission pathway, the agency provides significant detail about the study designs and comparator methods appropriate for evaluating quantitative, qualitative, and semi-quantitative tests.
As more and more tests are granted waived complexity status, clinical laboratorians may have some concerns about whether such tests are truly comparable to those performed in a CLIA-accredited facility by trained professionals. Those concerns can be addressed during the comment period for the two guidances, which FDA has extended until March 30, 2018. For lab professionals wanting to make their concerns known, now is the time to speak up.
References
- Select Updates for Recommendations for Clinical Laboratory Improvement Amendments of 1988 (CLIA) Waiver Applications for Manufacturers of In Vitro Diagnostic Devices: Draft Guidance for Industry and Food and Drug Administration Staff. Available at: www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm586506.pdf?utm_campaign=fda%20to%20extend%20comment%20period%20for%20draft%20guidances%20on%20clia%20waiver%20applications&utm_medium=email&utm_source=eloqua&elqtrackid=db9b0cc229fff4e77f4fdf76c76597dc&elq=b75539cdfc2b4eeea2861bb98473b9a8&elqaid=2181&elqat=1&elqcampaignid=1522. Accessed January 26, 2018.
- Recommendations for Dual 510(k) and CLIA Waiver by Application Studies: Draft Guidance for Industry and Food and Drug Administration Staff. Available at: www.fda.gov/ucm/groups/fdagov-public/@fdagov-meddev-gen/documents/document/ucm586502.pdf. Accessed January 26, 2018.
Steve Halasey
Chief Editor, CLP
[email protected]
(626) 219-0199