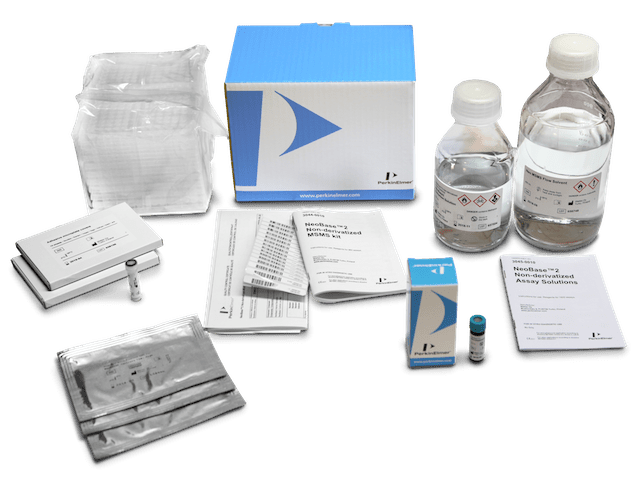
The NeoBase2 MS/MS kit can test for up to 57 analytes, including markers for screening of X-linked adrenoleukodystrophy, the most common peroxisomal disorder. It can also screen for adenosine deaminase severe combined immunodeficiency (ADA-SCID), which is caused by a deficiency of the enzyme ADA and is the second most common SCID.
The NeoBase2 MS/MS kit enables labs to use a simple three-step assay workflow to screen for disorders from a single dried blood spot punch. PerkinElmer’s optional MS/MS workstation software, which includes database functionality, helps labs effectively store, manage, review, and report results.
“As the industry leader in mass spectrometry-based newborn screening, we continue to evolve our technologies to meet the needs of laboratories worldwide, especially as more countries mandate certain metabolic tests such as those for SCIDs and peroxisomal disorders,” says Linh Hoang, MD, PhD, vice president for neonatal screening at PerkinElmer. “As these labs face pressure to screen for more disorders in less time and with limited resources, they are seeking advanced technology to expand their MS/MS testing capabilities.”
For more information, visit PerkinElmer.