A look at some of the exhibitors participating in the AACC 2006 Clinical Lab Exposition, July 25–27, in Chicago. | Sponsored section
|
Advanced Instruments’s Model 3320 Osmometer is designed to be an accurate and reliable instrument for osmolality measurement. The Model 3320 surpasses previous standards of performance for freezing-point measurement. A user-selectable, optional calibration routine optimizes the linearity in the instrument’s upper range (to 2,000 mOsm). This capability ensures testing accuracy and precision over the entire calibrated range. The instrument has a low-profile, compact enclosure for easy access to the sample probe and solenoid assembly. Its simple architecture eliminates sample errors, simplifies testing, and promotes maintenance-free operation. Other features include bar-code capability and electronic storage of results. |
|
The American Academy of Family Physicians presents The POL Microscopy Atlas, a benchtop reference manual for provider-performed microscopic examinations. The atlas includes more than 145 photographs of cellular elements, including urine sediments, vaginal wet preps, skin preps, nasal smears, stools for fecal leukocytes, stools for pinworms, and peripheral blood smears. Each photo has a matching description and clinical associations. The atlas also contains procedures for urine sediment and vaginal wet prep examinations and for peripheral blood smears, as well as the applicable CLIA regulations. Useful for training and continuing education, the atlas is approved for 8 prescribed hours of CME for physicians and 8 contact hours for lab personnel. |
|
American Diagnostica Inc The ACTICLOT® dPT™—a 510(k)-cleared dilute prothrombin time (dPT) test, when added to a lupus anticoagulant (LA) test panel, allows faster, more accurate detection of phospholipid-dependent LA autoantibodies. ACTICLOT dPT™ meets the test requirements of the recently published ISTH |
|
Antek HealthWare LLC Antek HealthWare LLC has provided software solutions for the health care industry since 1987. More than 30,000 physicians depend on Antek’s software to enhance productivity, improve patient care, and monitor their financial health. The LabDAQ laboratory information system remains the most widely used LIS with more than 1,800 installations in hospitals, reference labs, and physician’s office |
|
Bio-Rad Laboratories has introduced two new cardiac marker controls: Liquichek™ Cardiac Markers Plus Control LT and Liquichek™ Cardiac Markers Plus Control. These comprehensive products are made from human serum and can aid in monitoring a wide range of cardiac-assessment testing. Analytes include NT-proBNP, hs-CRP, troponin I, troponin T, CK-MB, myoglobin, homocysteine, total CK, and digitoxin. Both have a 3-year shelf life and a 20-day open-vial stability for all analytes. In addition, Cardiac Markers Plus Control LT features lower troponin targets and a fourth lower level. |
|
Pefakit® APC-R Factor V Leiden is a functional coagulation assay for activated protein C resistance that provides complete and distinct discrimination between the heterozygous, homozygous, and normal phenotypes caused by the Factor V Leiden mutation (FV:Q506). In contrast to other available methods, Pefakit APC-R Factor V Leiden shows no interference from factors upstream in the coagulation cascade, no interference from protein C or S, Lupus anticoagulants, elevated FVIII levels, heparins, or prothrombin G20210A. Pefakit APC-R FVL will create significant cost and time savings in the lab due to its accurate and reproducible determinations, all in an easy-to-use kit that is adaptable to semiautomated or automated coagulation analyzers. Validated methods for most coagulation analyzers are available upon request. |
|
Diagnostic Chemicals Ltd Diagnostic Chemicals Ltd (DCL) has added a new, fully liquid-stable, wide range C-reactive protein (CRP) assay to its already broad offering of clinical diagnostic products and services. The availability of DCL’s Wide Range CRP assay will benefit the needs of physicians, medical laboratory professionals, and clinical researchers alike. Using a highly sensitive immunoturbidimetric method, this two-part reagent |
|
Diamedix The Diamedix immunosimplicity® Epstein-Barr Virus (EBV) test kits are a panel of six ELISA tests for the detection of IgG or IgM anti-bodies to Viral Capsid Antigen (VCA), to early Antigen Diffuse (EA-D) and to Epstein-Barr Nuclear Antigen-1 (EBNA-1). These tests for the qualitative and semi-quantitative determination of antibodies to EBV have been manufactured to perform either manually or in |
|
The LIAISON® is a benchtop, random-access, chemiluminescent instrument that delivers the throughput capacity of floor-standing models. DiaSorin’s LIAISON system menu includes assays for infectious disease, bone and mineral metabolism, hypertension, autoimmunity, endocrinology, oncology, cardiac markers, sepsis, hepatitis, and fertility. LIAISON offers the only antibody-based 25-Hydroxyvitamin D assay available in an automated, random-access chemiluminescence platform along with Intact PTH assay. Laboratories benefit from LIAISON’s high throughput (180 tests per hour), rapid results (35 minutes to first result), assay reliability, and complete walkaway automation. |
|
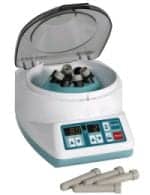
The Horizon Model 842VES, Plasmafuge-6, is the most compact of Drucker’s Performance Series centrifuges. The 842VES offers control over almost every aspect of the unit’s operation, from variable acceleration and deceleration to easy time and speed input. High speed and force capability produce platelet-poor/free plasma and greatly reduce processing times. The exclusive Drucker horizontal rotor allows for quick and easy sample loading and complete horizontal separation. The user can save settings in one of 10 memory locations and recall them at the touch of a button. |
|
Fujirebio Inc, the parent company of Fujirebio Diagnostics Inc, is the developer of the particle agglutination T. pallidum assay, SERODIA®TP•PA, which offers a convenient, sensitive, and specific gelatin particle-agglutination test for the detection of antibodies to Treponema pallidum. The SERODIATP•PA assay requires no special instruments, conjugate procedures, or equipment for preparation and running of tests. SERODIATP•PA can be used with serum or plasma (EDTA, heparin, or sodium citrate) with equally reliable results. It is easy to read and has clear cutoff values. SERODIATP•PA is distributed in the United States by Fujirebio Diagnostics Inc. |
|
The need for faster turnaround times and greater throughput can now be met with the new StatSpin Express 3 primary tube centrifuge. The fixed-angle rotor holds as many as eight blood tubes from 1.5 mL to 10.0 mL. The Express 3 can be placed next to any laboratory analyzer for stat chemistry, cardiac, or coagulation testing. Plasma or serum is obtained in as little as 2 minutes. The system is simple to use, and the small footprint frees up bench space so it can be used at the workstation. Tube inserts are available to accommodate a wide range of tube sizes. The powerful brushless motor is maintenance-free and is backed with a 2-year warranty. |
|
Following an extensive clinical study, Kronus has received 510(k) clearance from the US Food & Drug Administration for its glutamic acid decarboxylase (GAD) autoantibody test kit. The immunoassay test kit is for the semiquantitative determination of glutamic acid decarboxylase antibody in human serum. Measurement of antibodies to GAD is useful as an aid in the diagnosis and management of immune-mediated diabetes. |
|
Nanogen Nanogen’s CFTR ASRs*, the newest in the NGEN™ brand of molecular reagents, are primers and probes that target mutations associated with the cystic fibrosis gene. They can be used by laboratories to develop and optimize in-house test methods, such as population-carrier screening. A primer mix for the amplification of sequences for more than 600 CFTR mutations is available separately, and provides the laboratory with the flexibility to target the mutations they need for CFTR testing should they opt to develop tests to report more than the recommended 23 mutations. NGEN CFTR ASRs allow the laboratory to develop tests and to perform CFTR population-carrier screening in-house, while meeting the standard of care. Like all NGEN-brand reagents, these new CFTR reagents are specifically designed for PCR amplification, SNP genotyping, insertion/deletion detection, or pathogen-sequence detection by use of a two-color fluorescence-detection system. The product line includes reagents for the detection of mutations associated with Factor V/Prothrombin and Hereditary Hemochromatosis; and sequences associated with respiratory syncytial virus, influenza A and B, and parainfluenza 1, 2, and 3. |
|
Nova Biomedical The pHOx Plus L analyzer incorporates a liquid-only calibration system that eliminates bulky compressed tanks, gas regulators, gas tubing lines, and humidifiers. Moreover, a totally automated quality-control system is contained within a single onboard auto-cartridge QC pack. To promote blood conservation, pHOx Plus L measures the full 11-test menu with just 115 µL of whole blood, serum, or plasma and a three test micro-sample on just 55 µL. When used in conjunction with the Nova Point-of-Care Data Management System (PDM), pHOx Plus L can provide complete process automation from creation of test orders and accession numbers to interface with laboratory and hospital information systems for data capture. |
|
Orchard Software Nearly 600 laboratories across the country have turned to Orchard Software for their laboratory information systems—including all types and sizes of multisite and multispecialty clinics and physician’s office laboratories, hospitals, regional reference labs, student health services, and public health organizations. Orchard’s award-winning Harvest™ LIS uses process automation; robust instrument, |
|
Qiagen The Qiagen® BioRobot universal system is a complete automated solution providing the widest range of applications in 96-well format on one instrument. Gene expression and genotyping applications are available now, with sequencing and forensics applications to follow. The BioRobot Universal System enables fully automated purification of highly pure RNA from a range of sample types, including blood |
|
Quantimetrix Corp develops and manufactures innovative clinical diagnostic products for domestic and international distribution, including Lipoprint, the only FDA-cleared test intended to measure cholesterol levels in all lipoprotein fractions and subfractions. The Lipoprint system is a high-resolution diagnostic test for cholesterol components that are not routinely tested by other methods. The redominance of small dense low density lipoproteins (LDL) particles is associated with a three-fold increase of cardiovascular disease risk even at “normal” concentrations of LDL cholesterol. The Lipoprint test provides detailed results within the subfractions of LDL. The Lipoprint system is easy to use and can be installed at any clinical or research laboratory. |
|
|
|
Response Biomedical |
|
|

 Advanced Instruments Inc
Advanced Instruments Inc  The American Academy of Family Physicians
The American Academy of Family Physicians 
 laboratories. This Windows™-based LIS is an extremely flexible system, to grow and change with laboratories. LabDAQ3 is designed to effectively manage a laboratory’s data and resources. Key features include a comprehensive quality-control module, tools for medical-necessity compliance, a library of management and patient reports, security tools for HIPAA compliance, and connectivity to other systems (such as electronic medical records, HIS, and practice-management systems) or reference laboratories. LabDAQ offers consolidated and cumulative reports, extensive quality assurance, and sophisticated management tools. Options include bar code labeling, autoFAX, insurance filtering, e-mail distribution of results, a microbiology module, and DAQaccess for remote access to orders and results.
laboratories. This Windows™-based LIS is an extremely flexible system, to grow and change with laboratories. LabDAQ3 is designed to effectively manage a laboratory’s data and resources. Key features include a comprehensive quality-control module, tools for medical-necessity compliance, a library of management and patient reports, security tools for HIPAA compliance, and connectivity to other systems (such as electronic medical records, HIS, and practice-management systems) or reference laboratories. LabDAQ offers consolidated and cumulative reports, extensive quality assurance, and sophisticated management tools. Options include bar code labeling, autoFAX, insurance filtering, e-mail distribution of results, a microbiology module, and DAQaccess for remote access to orders and results.  Bio-Rad Laboratories Inc
Bio-Rad Laboratories Inc  Centerchem Inc
Centerchem Inc  system quantitatively and accurately determines concentrations of CRP in serum over a wide range of clinically significant concentrations. DCL’s Wide Range CRP reagent incorporates a highly purified monoclonal murine antihuman CRP antibody bound to latex beads, which provides an extended reportable range from 0.01 to 36.0 mg/dL without compromise to low-end sensitivity or precision. DCL’s Wide Range CRP assay demonstrates no significant interference effects from lipemia, hemolysis, and ascorbic acid; and maintains long-term, reliable performance characteristics. A variety of kit-packaging configurations is available, making it well-suited for use in hospital, reference, and physician’s office laboratories, regardless of workflow demands. DCL’s Wide Range CRP is optimized for use with fully automated testing systems, and a comprehensive listing of instrument applications is readily available for a number of chemistry analyzers. Additional calibration materials are also available to fully support the assay.
system quantitatively and accurately determines concentrations of CRP in serum over a wide range of clinically significant concentrations. DCL’s Wide Range CRP reagent incorporates a highly purified monoclonal murine antihuman CRP antibody bound to latex beads, which provides an extended reportable range from 0.01 to 36.0 mg/dL without compromise to low-end sensitivity or precision. DCL’s Wide Range CRP assay demonstrates no significant interference effects from lipemia, hemolysis, and ascorbic acid; and maintains long-term, reliable performance characteristics. A variety of kit-packaging configurations is available, making it well-suited for use in hospital, reference, and physician’s office laboratories, regardless of workflow demands. DCL’s Wide Range CRP is optimized for use with fully automated testing systems, and a comprehensive listing of instrument applications is readily available for a number of chemistry analyzers. Additional calibration materials are also available to fully support the assay.  conjunction with the company’s automated EIA systems. The tests provide an antibody profile, which can be of diagnostic importance in the assessment of potential EBV-related infectious mononucleosis. Color-coded reagents with matching caps are ready for either manual or automated use. Results are obtained within 2 hours, with just 15 minutes hands-on time. The kit’s standard 96-microwell-plate format adapts well to large-volume laboratories, while its breakapart strip format provides small-volume flexibility. The tests can be batch-tested concurrently with other EIA assays. The end products are measured spectrophotometrically, yielding objective results that denote antibody presence/absence in the sample. Results of well-characterized sera indicated that 94% to 100% of seronegative sera were negative in all tests in the panel. Sera from current infections were positive for VCA IgM (95%), VCA IgG (85%), and EA-D (40%), while predominantly (87%) negative for EBNA IgG. Convalescent (past infection) sera were negative for VCA IgM (97%) and EA-D IgG (90%), and positive for EBNA IgG (98%) and VCA IgG (100%).
conjunction with the company’s automated EIA systems. The tests provide an antibody profile, which can be of diagnostic importance in the assessment of potential EBV-related infectious mononucleosis. Color-coded reagents with matching caps are ready for either manual or automated use. Results are obtained within 2 hours, with just 15 minutes hands-on time. The kit’s standard 96-microwell-plate format adapts well to large-volume laboratories, while its breakapart strip format provides small-volume flexibility. The tests can be batch-tested concurrently with other EIA assays. The end products are measured spectrophotometrically, yielding objective results that denote antibody presence/absence in the sample. Results of well-characterized sera indicated that 94% to 100% of seronegative sera were negative in all tests in the panel. Sera from current infections were positive for VCA IgM (95%), VCA IgG (85%), and EA-D (40%), while predominantly (87%) negative for EBNA IgG. Convalescent (past infection) sera were negative for VCA IgM (97%) and EA-D IgG (90%), and positive for EBNA IgG (98%) and VCA IgG (100%).  DiaSorin Inc
DiaSorin Inc  The Drucker Co
The Drucker Co  Fujirebio Diagnostics Inc
Fujirebio Diagnostics Inc Iris Processing Inc
Iris Processing Inc  Kronus Inc
Kronus Inc 
 (SO2%), hematocrit, and hemoglobin in addition to the standard blood gas menu of pH, pCO2, and pO2. The pHOx Plus L also incorporates glucose, lactate, sodium, potassium, and a choice between ionized calcium or chloride to offer the essential test menu for the ICU, OR, ED, or stat laboratory.
(SO2%), hematocrit, and hemoglobin in addition to the standard blood gas menu of pH, pCO2, and pO2. The pHOx Plus L also incorporates glucose, lactate, sodium, potassium, and a choice between ionized calcium or chloride to offer the essential test menu for the ICU, OR, ED, or stat laboratory.  billing, HIS, EMR, and reference lab interfaces; and rules-based technology to address regulatory and integration issues and simplify laboratory workflow. Orchard Harvest LIS improves reimbursements and simplifies the time-consuming task of medical-necessity validation by automatically screening ICD-9 codes, testing frequency, and experimental procedures during order entry. As an HL7 organization member and a stand-alone lab system, Orchard specializes in seamless integration, which makes host system, EMR, billing, and reference-lab interfaces routine, and linking multiple sites possible. Orchard’s Microbiology and Anatomic Pathology Modules eliminate paper and electronically integrate these departments with the rest of the laboratory. For remote order entry and results delivery via the Internet, Orchard offers Webstation™.
billing, HIS, EMR, and reference lab interfaces; and rules-based technology to address regulatory and integration issues and simplify laboratory workflow. Orchard Harvest LIS improves reimbursements and simplifies the time-consuming task of medical-necessity validation by automatically screening ICD-9 codes, testing frequency, and experimental procedures during order entry. As an HL7 organization member and a stand-alone lab system, Orchard specializes in seamless integration, which makes host system, EMR, billing, and reference-lab interfaces routine, and linking multiple sites possible. Orchard’s Microbiology and Anatomic Pathology Modules eliminate paper and electronically integrate these departments with the rest of the laboratory. For remote order entry and results delivery via the Internet, Orchard offers Webstation™.  (collected in PAXgene™ Blood RNA Tubes), tissues, and cells, plus RT-PCR setup in 96-well format. The system also enables purification of high-quality genomic DNA from up to 192 buccal swab samples or up to 96 blood samples. Fully automated protocols provide reliable and flexible PCR and RT-PCR setup in 96-well format. Highly standardized processing enables reproducible results between experiments and labs. Automated reaction setup provides high levels of reliability and helps to eliminate variation caused by handling errors, particularly when processing a large number of samples. With the BioRobot Universal System, highly reproducible CT values are achieved—with no detectable cross-contamination—demonstrating the efficiency and reliability of the automated procedure. QIAsoft 5, the controlling software for the BioRobot Universal System, enables users to comply with the technical requirements of 21 CFR Part 11. In addition, files used or generated by QIAsoft 5 are in XML format, enabling easy data management and compatibility with laboratory information management systems (LIMS). QIAsoft 5 also allows easy setup of pipetting schemes for customized applications.
(collected in PAXgene™ Blood RNA Tubes), tissues, and cells, plus RT-PCR setup in 96-well format. The system also enables purification of high-quality genomic DNA from up to 192 buccal swab samples or up to 96 blood samples. Fully automated protocols provide reliable and flexible PCR and RT-PCR setup in 96-well format. Highly standardized processing enables reproducible results between experiments and labs. Automated reaction setup provides high levels of reliability and helps to eliminate variation caused by handling errors, particularly when processing a large number of samples. With the BioRobot Universal System, highly reproducible CT values are achieved—with no detectable cross-contamination—demonstrating the efficiency and reliability of the automated procedure. QIAsoft 5, the controlling software for the BioRobot Universal System, enables users to comply with the technical requirements of 21 CFR Part 11. In addition, files used or generated by QIAsoft 5 are in XML format, enabling easy data management and compatibility with laboratory information management systems (LIMS). QIAsoft 5 also allows easy setup of pipetting schemes for customized applications.  Quantimetrix Corp
Quantimetrix Corp  Randox Laboratories Ltd
Randox Laboratories Ltd 
 Wescor Inc
Wescor Inc