
Like dedicated middleware, SCC Soft Computer’s SoftLab provides robust utomation/rules-related functionality in analytical stages and for QC.
Today, there seems to be a whirlwind of discussion surrounding the topic of “middleware,” says Curt Johnson, COO, Orchard Software, Carmel, Ind.
“Notice that the term ‘middleware’ is in quotes,” he says. “The reason for this is that middleware is somewhat nebulous in its meaning, as it is used to refer to numerous systems, each performing their own unique tasks and used by a variety of laboratories of various types and sizes. And ARRA/HITECH has been one of the reasons for some of the increased interest.”
Middleware—computer software that connects software components or applications—is, as the name implies, software in the middle; in laboratory usage, it’s a product that is interposed between the laboratory information system (LIS) and the lab analyzers that do the actual testing; it serves as an interface but can also offer additional functionality. Middleware allows multiple processes running on one or more machines to interact across a network.
But some middleware now serves as a rules engine that can process test results coming from the lab instrument. In fact, middleware has grown vastly more complex, and its function and role as a facilitator has expanded so greatly that there is some disagreement over how to define it.
Don Keller, vice president of education/training, SCC-Soft Computer, Clearwater, Fla, says that so-called “middleware” was originally developed as a means of connecting instruments to a computerized system (LIS or otherwise). Communication from the instrument often had to be “translated” in order for it to be received by an LIS or other application. As it evolved, “middleware developers began to incorporate features that were missing from many laboratory information systems, particularly with HIS-based LIS or lower-end LIS systems. Such features generally include a rules engine—or decision-support tools—to help automate the analytical phase of testing,” Keller says.
But in essence, Keller says, a middleware application is a PC/server system that resides between a lab instrument and an LIS. One could consider a POCT data manager as a form of middleware. Today, middleware developers have begun to incorporate additional features that have been traditionally viewed as instrument technical support systems such as quality control (QC) and remote instrument diagnostics. The challenges for many laboratories (eg, limited budget for a more robust LIS, or hands tied by admin/CIO) can be resolved by using a middleware package as a “work-around” to deliver to the laboratory the tools they need without necessarily involving IT. Even today, some LISs are deficient in the area of rules, instrument management, and QC, and must rely on middleware systems.
Middleware, from a laboratory perspective, has evolved dramatically in the past few decades. The laboratory informatics market, since its inception, has required both instrument and systems integration. “This was driven by both the process and life science industries,” says David Minicuci, director, field marketing, Informatics and Laboratory Automation, Thermo Fisher Scientific, Philadelphia. “Today’s economic climate elevates this need even further since the real value for a business to invest in an informatics solution is the up and downstream systems connectivity, which not only provides automation but facilitates the aggregation of information across the business. This deployment model will not only leverage lab information into business value, it will also support the rapid delivery of results from the lab directly to the patient’s bedside.”
To meet the needs of today’s complex clinical laboratory integration challenges, Thermo Fisher Scientific introduced a Clinical LIMS at the American Association for Clinical Chemistry (AACC) annual meeting in July, which integrates with a middleware application called Integration Manager. (For more information, see CLP ’s September issue, Product News section) Integration Manager is a comprehensive solution that utilizes XML transform technology complete with an intuitive user interface that provides an automated solution for laboratory connectivity to both instruments and enterprise level applications such as EMR, ERP, MES, and CRM solutions. Once configured the process of data transfer is rapid and consistent. Integration Manager provides a configurable, extensible, and easily managed solution for laboratory and enterprise connectivity.
Lisa Jean Clifford, CEO, Psyche Systems Corp, Milford, Mass, says that middleware in the health care industry is simply defined as software that sits on the bridge between laboratory instruments and the LIS and is integrated to both. The software helps automate the laboratory by providing benefits from speed and accuracy to efficiency. Clinical labs rely on the middleware to provide automatic validation and reporting of normal results, and to track and manage the tests and online activity of all instruments simultaneously in the lab. Middleware functionality can be built directly into the LIS or integrated with it through third-party applications.
Regardless of the size or type of the lab, middleware can be a valuable asset if it is integrated with the LIS properly. Many labs make the mistake of thinking that middleware can replace the capabilities of an LIS altogether. A properly utilized middleware solution should be almost invisible to the end user. Middleware can streamline the complex processing of autoverification, and integrate top-end quality control processing and cost-effective “off-the-shelf” instrument interfaces.
Some middleware companies are trying to expand the scope of their focus to include integration with EMRs and practice-management systems. While on the surface this seems like a natural evolution of integration, in reality, EMR systems are grossly more complex and very different from clinical instrumentation, and this fact should be carefully considered before relying on a middleware solution to help labs achieve their current and, more importantly, future integration requirements, Clifford says.
Dianna Powell, presales clinical consultant, LIS, Merge Healthcare, Chicago, says that LIS middleware is software that moves data from one device or system to another, and is used to convert or translate data into a format (ie, HL7) that is understandable by the receiving system, usually an EMR or LIS/LIMS. “Ideally, the middleware would also provide logic rules and algorithms that allow results that meet acceptable limits to pass through to the receiving system automatically, while results that don’t ‘pass’ are queued for review by a technologist,” Powell says. “Middleware is vital to clinical labs because it interfaces data that would otherwise be transcribed manually (via direct entry or through scanning of documents) and automates, organizes, and simplifies the data transfer. Medical practices that wish to qualify for meaningful-use reimbursements must automate the electronic transfer of medical data and middleware products can help achieve this mandate.”
And middleware satisfies needs where traditional LIS systems fail, says Douglas Matthews, senior clinical marketing manager, bioMérieux Inc, Durham, NC. Workflow optimization, remote instrument monitoring, and efficient data integration are commonly expressed needs in the lab community. Middleware is an intermediary device that facilitates communication among disparate systems, such as lab analyzers, and the LIS. Middleware can be as simple as a serial connected network appliance focused on instrument connectivity, or as complex as a Web server that consolidates interfaces to multiple instruments through the institution’s network.
To better define and summarize middleware, its various uses, who is most likely to use it, and how it might relate to HITECH, Johnson broke it into four possible categories:
MIDDLEWARE USED BETWEEN THE LIS AND ANALYZERS OR AUTOMATION LINES
Middleware used between the legacy LIS and analyzers or automation lines is probably the oldest, most original source of the term middleware and is used primarily by large, high-volume hospital or reference laboratories. Middleware systems used in these scenarios are typically developed and supplied by the instrument or automation line vendor to facilitate the use of the instrument or automation line, and/or to supplement their use with the limited capabilities within the legacy LIS. The middleware will link multiple analyzers, perform special rules, or guide the automation line, and the determination for its use and a return on the investment is dependent on the needs of the lab and the capabilities of the existing LIS, Johnson says.
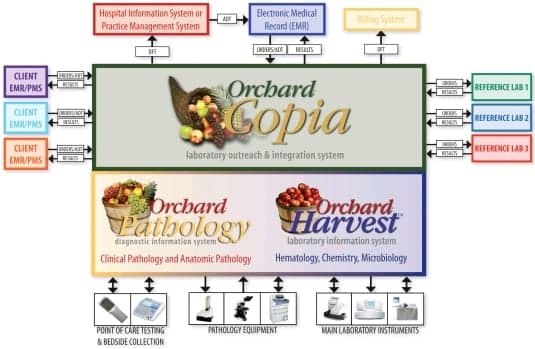
MIDDLEWARE USED AS A LINK TO THE LIS FROM OUTSIDE THE LAB
A second form of middleware appeared about 10 years ago to provide a link to LIS from remote locations, Johnson says. Initially and prior to the increased adoption of the electronic medical record (EMR), this middleware was a Web-based lab portal that provided remote access to the LIS for order entry and result look-up. Today, with more and more EMRs in place, the lab portal middleware has evolved into an integration tool electronically linking remote lab clients’ EMRs directly to the lab’s LIS. This provides the seamless transmission of lab orders from the EMR to the LIS and results back from the LIS to the lab clients’ EMR. Orchard Copia and Orchard Harvest Webstation are both integration systems that provide this type of seamless integration between the LIS and remote EMRs, Johnson says.
While this type of middleware has been around for some time, it has been ARRA/HITECH that has recently increased the demand for these types of integration systems. The goal of HITECH is to stimulate EMR adoption and its use with the ultimate outcome being the elimination of paper records and providing the ability to store, structure, share, and analyze medical data to improve patient care. A large portion of medical data used, stored, and analyzed for patient care is lab results, Johnson says.
“And as part of proving Meaningful Use, to qualify for and receive the financial incentives offered by the government, the ability to transmit lab results is one of the established criteria,” he continues. “While reference laboratories do not use EMRs themselves, it is their clients who have an EMR who look to qualify for the financial incentives, and who demand the electronic integration. Today, to remain competitive, most reference laboratories or hospitals performing outreach testing must have a middleware system providing those direct LIS-to-EMR interfaces.”
MIDDLEWARE USED BETWEEN ANALYZERS AND THE EMR
Johnson says middleware used between analyzers and the EMR is a recently new form of middleware and is primarily used by very small physician’s office laboratories as an inexpensive alternative to manual entry to get lab results from the instruments into the EMR. HITECH has been the primary stimulus for this type of middleware to help physicians meet Meaningful Use criteria. While this type of middleware offers a less expensive alternative to a traditional LIS, it does not provide the benefits of a small LIS that also performs and enhances laboratory work and workflow, such as billing, delta checking, autoverification, quality control (QC), quality assurance (QA), and the necessary CLIA benchmarking. If a facility is considering this type of middleware purchase, consideration of the added benefits of a small LIS might provide a more compelling return on the larger investment, Johnson says.
MIDDLEWARE TO AUGMENT MOLECULAR TESTING AND RESULT REPORTING
Finally, Johnson says, another new form of middleware is being used by sophisticated laboratories for complex molecular testing and the reporting thereof. This type of middleware, typically highly specialized, complex, and customized, is attached to the analyzer and gathers, analyzes, and applies complex algorithms on the results to generate the report data. This middleware may then be linked to LIS or a centralized database for results storage or additional analysis, making it easy for the end user to look up the results and mine them for future studies, he says.

Psyche Systems offers a complete LIS for both anatomic and clinical pathology: its e.lixa suite of modules for microbiology, outreach, and EMR integration.
Clifford says that, “in general, labs are looking for their LISs to become more robust, while at the same time increasing their ‘Lean-ness.’ Many LIS vendors are currently faced with scrapping their entire platform in order to compete with the changing needs of the clinical lab, particularly when you consider the importance of diagnostics in the field of pathology. “Labs need their LIS to handle everything from increased testing menus to outreach to client service,” Clifford adds. “Middleware is only one part of this equation. Psyche Systems Corp offers a complete LIS for both anatomic and clinical pathology—our e.lixa suite of modules for microbiology, outreach, and EMR integration, all fully integratable with each other, instrumentation, or any other vendor’s application.”
According to Keller, the decision to purchase an LIS—or incorporate a middleware system alongside an LIS—may or may not be a decision the laboratory can make without support or approval from IT or administration. Getting support for the endeavor is an important step, whether big or small.
“Challenges include implementing the system, which will involve interfaces to and from instruments, determining what the LIS will do and what the middleware will do, the system build/setup, and extensive validation of the systems,” Keller says. “With instruments, many now have output that includes images and graphs, which many laboratory information systems cannot receive, even if the middleware can. When automating, new workflows must be engineered for testing and QC, new SOPs created, etc.”
He adds that keeping the project on time and on budget can also be a challenge if the laboratory has not been involved with IT projects in the past. Unified specimen tracking can also be a challenge because the middleware may not know the origin of the sample, or what happens to the sample afterward.
Keller notes that SCC Soft Computer’s SoftLab® LIS is among the most robust in the industry. SoftLab is able to directly interface to instruments, receive all data and images, and display them directly on-screen (no need to go to the instrument to see graphs/images). “Like dedicated middleware, SoftLab provides robust automation/rules-related functionality in analytical stages and for QC. In addition, SoftLab incorporates rules and automation tools during preanalytical and postanalytical processes,” Keller says. “The ability to actually graph and build workflows and rules is quite enhanced in SCC’s Genetics Information Systems Suite™ of diagnostic genetics laboratory information management applications as those instruments can be quite complex, involving both data and images, and often producing diagnostic results. The incorporation of genetics lab instruments and testing is an area that is presenting challenges for many labs.”
Keller says SCC adapted and evolved its SoftLab LIS and Genetics Suite applications to enable them to connect directly to instruments and via Web Services-based interfacing—and provide the ability to collect and view all output from an instrument directly on the LIS screen, even multiple instruments—on a single screen. SCC also developed a comprehensive QC information system, SoftTotalQC®, which is a global QC system that goes across all disciplines within the LIS, and can even allow the performance of QC on medical equipment not “on the line,” such as refrigerators. Such equipment can be bar coded and QC performed by a user with a wireless handheld PC device. This allows centralization of all lab QC, which is quite optimal for reporting across the various lab departments (centralized QC data for the entire lab).
MIDDLEWARE IN SMALL/LARGE LABS
For the small laboratory, says Powell of Merge, middleware offers the ability to interface a stand-alone hematology and/or chemistry analyzer to a new EMR system while mitigating the need to invest in a comprehensive LIS. “This allows the smaller physician office lab that is deploying a new EMR with reference lab interfaces, etc, to connect its local in-house lab to the EMR too,” Powell says. “Small labs tend to have limited IT resources and laboratory staff available, so a ‘good-fit’ middleware solution will be one that is simple to deploy and easy to configure.”
In the large laboratory with high-priced LIS/LIMs, she says, simply changing out an analyzer can be quite expensive due to interface costs. Middleware can make one analyzer look and communicate like another analyzer to an existing LIS/LIMs system, at a very economical price. Also, for the large lab with satellite locations, middleware can offer a connectivity bridge to remote instruments and devices. The key to successful middleware deployment for the large lab is a clearly defined set of objectives and selecting the right product for the project scope.
Merge LabAccess serves the small waived and moderately complex physician’s office lab market. LabAccess provides economical connectivity between a lab’s analyzers and the practice EMR, and supports multiple instrument interfaces, manual entry of offline results, user approval of results with comments, consolidated patient reports, and bidirectional HL7 EMR interfaces.
“Currently, the development requests we receive are for the creation of HL7 Orders and Results Interfaces,” Powell says. “LabAccess offers a Scripting Engine for our HL7 Message Content as well as multiple file transport plug-ins, providing our implementation team with the tools to quickly and efficiently respond to new development requests.”
bioMérieux’s Matthews says laboratories are struggling to cope with increasing test volumes while striving to improve patient care. Challenges arise from the loss of skilled resources, increased oversight, decreased budget, and the overall need to do more with less. In striving to become Lean and efficient, many laboratories are incorporating middleware solutions. LIS and middleware vendors are increasing the functionality of their product offerings to promote more efficient workflow, which reduces turnaround time of lab results and increases the usability of their systems.
Matthews says the degree of difficulty in implementing a middleware solution depends upon the complexity of the solution. With solutions focused upon interfacing, the lab’s LIS coordinator typically handles the installation of the device through a terminal server. The device itself usually requires little programming, and both the acquisition and maintenance costs are nominal. Most of these installations do not require extensive involvement from the information technology or information systems departments.
With solutions that require instrument connectivity through the network or the Internet, IT/IS project approval is required. Therefore, it is a good idea to get them involved early in the process to prevent delays. Implementation kickoff meetings should address topics such as virus protection, data backups, and protection of patient information in keeping with HIPAA requirements. Early IT/IS buy-in is crucial to keeping the project on track, and ensures that the lab receives the proper internal support, Matthews says.
Myla™ is an innovative microbiology middleware solution from bioMérieux that focuses on consolidated interfacing, workflow optimization, and information management. Myla is Web-based and consolidates information that can impact patient care and efficiency from ID/AST and blood culture testing in a single interface for the LIS. Users can access Myla remotely from anywhere on their network. The intuitive dashboard allows users to rapidly gain a comprehensive view of their lab’s blood culture process, perform remote validation of identification and susceptibility results, and easily pull reports.
Myla also provides more visibility for technologists to help them take control of the testing process and improve the information available to the clinician. For instance, anyone logged into Myla is notified when blood cultures become positive, and alerts can be delivered via e-mail or text messages. Lab managers can quickly adjust their workflow around positive blood cultures and the related workups to deliver clinically relevant information.
Gary Tufel is a contributing writer for CLP.



