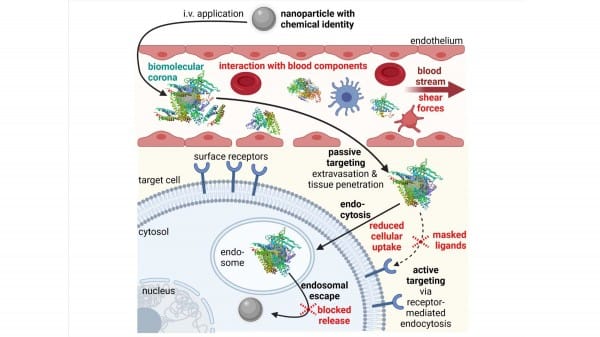
Researchers have published a study regarding the design and application of nanoparticles as efficient delivery vehicles for biopharmaceutics, after observing a weak correlation between in vitro and in vivo performance for drug delivery research. When nanoparticles are applied intravenously, they face several obstacles that differ from in vitro situations, such as when they meet blood components. Nanoparticles are usually covered by a biomolecular multilayer (a protein corona), which alters the physiochemical properties, pharmacokinetics, and toxicity profile of the nanoparticles.
In Biophysics Reviews, from AIP Publishing, researchers in Germany provided a new characterization of the protein corona formed around nanoparticles and its impact on the physiochemical and biological properties of these nanoparticles.
“When predicting in vivo performance from in vitro data, it is recommended to combine several analytical and biological characterization methods to get more detailed insight into the in vivo characteristics and behavior of the nanoparticles,” says Simone Berger, a co-author from Ludwig Maximilian University of Munich.
The choice of the biofluid—serum, plasma, or full blood, and animal in origin—and establishment of standardized protocols are important for more consistent, robust, and comprehensive preclinical studies to derive structure-activity relationships and in vitro/in vivo correlations.
“The knowledge gained about protein corona formation can be exploited to optimize carriers for nanomedical application,” says Berger.
Information like in vivo biodistribution and off-target effects cannot be obtained from in vitro experiments, the researchers say. But new high-throughput screening methods like the barcoding system can make in vivo investigations more effective, economical, and ethical.
Nanomedicine shows “great potential to revolutionize the therapeutic landscape with a broad range of applications like cancer vaccines/immunotherapy or treatment of genetic disorders,” says Berger. “With proper and more predictive in vitro assays, the preclinical pipeline will become more efficient, faster, and economic. And importantly, animal experiments can be replaced or at least reduced.”
Featured image: Obstacles (in red) in the in vivo delivery process of intravenously (IV) applied nanoparticles. Photo: American Institute of Physics (AIP)