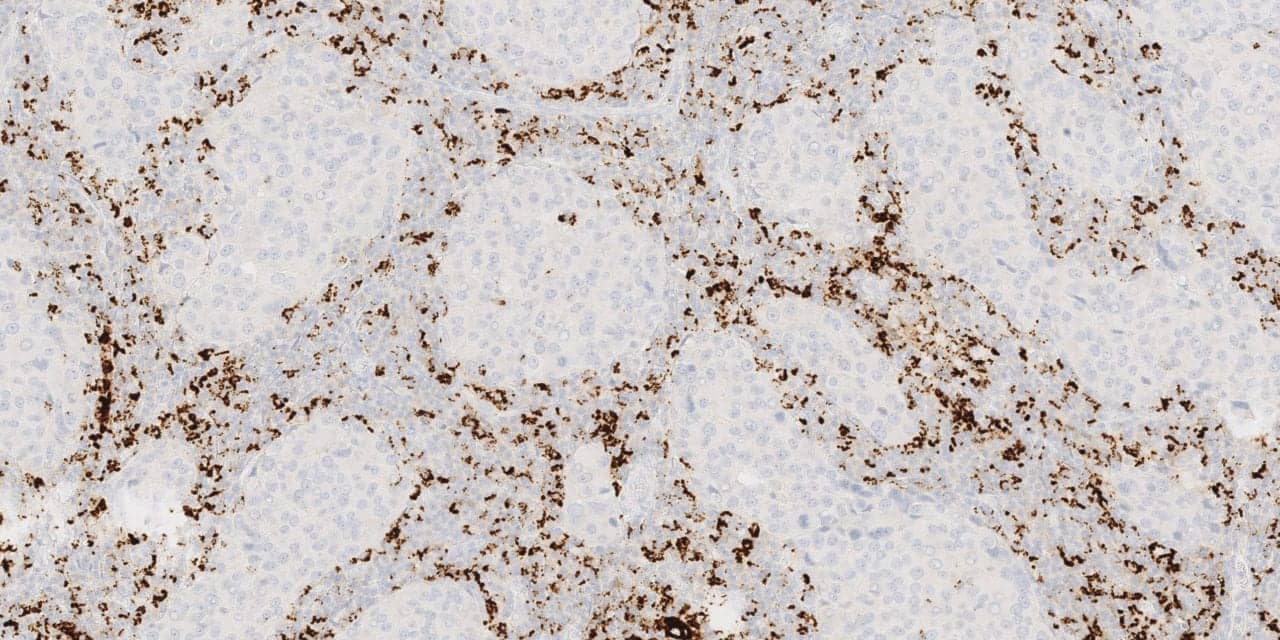
Roche Diagnostics, Basel, Switzerland, has expanded the use of its Ventana PD-L1 (SP 142) assay for patients undergoing treatment for triple-negative breast cancer in regions where the CE-marked Roche cancer immunotherapy medicine atezolizumab (Tecentriq) is available. The Ventana PD-L1 assay is the first companion diagnostic to aid in identifying triple-negative breast cancer patients eligible for treatment with Tecentriq plus chemotherapy (nab-paclitaxel).
Each year, 300,000 patients around the world are diagnosed with triple-negative breast cancer, an aggressive disease with limited treatment options.1,2 A diagnosis of triple-negative breast cancer means that the three most common proteins associated with breast cancer growth—estrogen receptor, progesterone receptor, and HER2/neu—are not expressed on the tumor.
Roche says the launch of its Ventana PD-L1 (SP142) assay represents an important step in its personalized healthcare strategy to fit treatments to patients who can benefit most from a specific medicine. Using the assay to assess the status of the PD-L1 biomarker on tumor-infiltrating immune cells is essential for identifying such patients, according to the company. The announcement follows FDA’s March approval of the assay as the first companion diagnostic to identify triple-negative breast cancer patients eligible for the Tecentriq combination.
“Until recently, the only treatment option for metastatic triple-negative breast cancer patients was chemotherapy,” says Thomas Schinecker, CEO of Roche Diagnostics. “With our expanding menu of companion diagnostics and targeted cancer immunotherapies, Roche is proud to continue to deliver on our mission to make personalized healthcare a global reality, ensuring the right treatment for the right patient at the right time.”
The Ventana PD-L1 (SP142) assay was developed to enhance the visual contrast of tumor-infiltrating immune cell staining. In triple-negative breast cancer, PD-L1 is primarily expressed on tumor-infiltrating immune cells rather than on the tumor cells themselves.
Launched in 2016, the Ventana PD-L1 (SP142) assay is the primary diagnostic assay within the Tecentriq clinical development program and was used to enroll and stratify patients in Tecentriq clinical trials. It was the enrollment assay used in the Impassion 130 trial, the first positive Phase III immunotherapy regimen study in triple-negative breast cancer. The assay was the first to evaluate patient PD-L1 biomarker status using immune cell staining and scoring within the tumor microenvironment.
The PD-L1 (SP142) assay is proven to identify patients most likely to respond to treatment with Tecentriq, as demonstrated by higher overall response rates in Cohort 2 of the Imvigor 210 clinical trial. The novel approach uses immunohistochemistry (IHC) technology designed to visually enhance and score PD-L1 protein on tumor-infiltrating immune cells. In an analysis based on 14.4 months of median follow up, Tecentriq shrank tumors in 15% (95% CI: 11, 19) of people evaluable for efficacy whose disease progressed after platinum-based chemotherapy (n = 310). Tecentriq shrank tumors in 26% (95% CI: 18, 36) of people whose disease had medium and high levels of PD-L1 expression (n=100).
The Impassion 130 study is a Phase III, multicenter, randomized, double-blind study. It is evaluating the safety, efficacy, and pharmacokinetics of Tecentriq plus nab-paclitaxel compared with placebo plus nab-paclitaxel in people with unresectable locally advanced or metastatic TNBC who have not received prior systemic therapy for metastatic breast cancer.
Tecentriq is a cancer immunotherapy that has the potential to be used as a foundational combination partner with other immunotherapies, targeted medicines, and various chemotherapies across a broad range of cancers, according to Roche. The monoclonal antibody is designed to bind with the PD-L1 protein, which is expressed on tumor cells and tumor-infiltrating immune cells, blocking its interactions with both PD-1 and B7-1 receptors. By inhibiting PD-L1, Tecentriq may enable the activation of T cells. The immunotherapy is approved in the United States, the European Union, and other countries. It can be used either alone or in combination with targeted therapies.
Available on the automated BenchMark IHC/ISH series instruments, the Ventana PD-L1 (SP142) assay uses the OptiView amplification kit and the OptiView DAB IHC detection kit. The assay performs specific staining of tumor cells and immune cells. It is approved for use in the United States and European Union as a companion diagnostic in urothelial carcinoma and as a predictive assay for the use of Tecentriq in non-small cell lung cancer.
For more information, visit Roche.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424; doi: 10.3322/caac.21492.
- Eliyatkin N, Yalçin E, Zengal B, Aktas S, Vardar E. Molecular classification of breast carcinoma: from traditional, old-fashioned way to a new age, and a new way. J Breast Health. 2015;11(2):59–66; doi: 10.5152/tjbh.2015.1669.