For patients with metastatic hormone receptor-positive (HR+) breast cancer, there may be no test more important than monitoring for an ESR1 mutation.
By John McNamara and Tom Bittick
With so many types of breast cancer, there are already a plethora of tests that clinical laboratories must be familiar with to meet the needs of this sizable patient population. But for patients with metastatic hormone receptor-positive (HR+) breast cancer, there may be no test more important than monitoring for mutations in the ESR1 gene.
While ESR1 mutations are extraordinarily rare in treatment-naïve patients, it’s a very different story once treatment begins. Patients who receive first-line aromatase inhibitors to prevent the conversion of androgens to estrogens are at risk of developing these mutations, with as many as 40% of patients acquiring ESR1 mutations during the course of treatment1. Some of these mutations can be harmless but others drive significant risk of treatment-resistant disease recurrence. Certain ESR1 mutations constitutively activate the pathway that the cancer needs to grow, allowing it to slip past the defenses offered by endocrine therapy and surge back to full strength.
That’s why ESR1 mutation monitoring is essential to staying one step ahead of the cancer. Regular testing—as often as every other month—allows clinicians to spot mutations early and adjust treatment before the cancer has had the opportunity to recur. Now, this monitoring is becoming more important than ever with the development of targeted therapies designed to suppress the effects of ESR1 mutations for patients with breast cancer.
Promising Clinical Trials
Recently, clinical trials have demonstrated the potential for new ESR1 mutation-targeting therapies to make cancers susceptible to endocrine therapy again.
The PADA-1 study, for example, is a randomized, multi-center, phase 3 trial that launched in France in 20172. It enrolled more than 1,000 HR+ metastatic breast cancer patients and was designed to evaluate the outcome of switching patients who develop ESR1 mutations from aromatase inhibitors to fulvestrant, a therapy known to be more effective against certain ESR1 mutations. Patients were shifted to fulvestrant for two reasons: either early detection of a new ESR1 mutation picked up by genetic testing conducted every two months, or imaging-based tumor progression indicating that the standard treatment was no longer effective.
While the PADA-1 trial is not expected to be completed until 2025, interim results have been exciting. In patients who were switched to fulvestrant when ESR1 mutations were first detected, median progression-free survival more than doubled compared to patients who remained on the standard treatment3,4. It was the early intervention that made the difference; patients who switched to fulvestrant at a later stage, when tumor progression was sizable enough to be detected by imaging, saw minimal benefit in progression-free survival.
This clinical trial design has been replicated in the newer SERENA-6 study, a global phase 3 multi-center trial designed to enroll 300 patients5. The trial is testing camizestrant, a next-gen candidate treatment from AstraZeneca expected to be more effective against cancer with ESR1 mutations. Results from an earlier phase 2 study of this treatment were presented at the San Antonio Breast Cancer Symposium in 2022, with progression-free survival nearly tripling for patients with ESR1 mutations6. Based on this early data, camizestrant appears to have broad efficacy against all ESR1 mutations in the genomic region.
ESR1 Mutation Testing Today
In addition to demonstrating the effectiveness of alternative therapies for patients who develop ESR1 mutations, these clinical trials also clearly indicate that routine testing for ESR1 mutations will be critical for ensuring better outcomes for these breast cancer patients going forward.
Unfortunately, detecting these mutations in DNA that comes only from tumors requires tremendous sensitivity. A liquid biopsy approach would be ideal given the frequency of testing desired, but the conventional molecular testing platforms used for circulating tumor DNA (ctDNA) in blood samples struggle to achieve the necessary level of sensitivity.
Today, many clinical laboratories address this problem by sending out ESR1 mutation tests to specialty commercial labs. There are two major drawbacks to this model. First, send-out testing may be a good option for one-off tests, but for a test that has to be completed bi-monthly for each patient, it makes less economic sense. Send-out testing is typically more expensive than a test run in the clinical lab and bringing the test in-house is more fiscally responsible for both the laboratory and for the patient. The second issue is turnaround time: Send-out tests usually take longer to return results. As the clinical trials mentioned above are demonstrating, the risk of progression is likely to increase the longer patients remain on standard therapy after acquiring an ESR1 mutation. Testing performed at the clinical lab would make it possible to shift patients to an ESR1-targeted treatment—or another alternative treatment—as soon as possible, perhaps even before the cancer has had a chance to progress.
It’s easy to say that ESR1 mutation testing should be brought in-house, but it is understandably harder to achieve. In the PADA-1 clinical trial, ESR1 mutation testing has been performed with a niche droplet digital PCR platform. Digital PCR overcomes the sensitivity limitations of other technology platforms, but it is not yet commonplace in clinical laboratories due to limited test menu and the additional equipment needed to implement it. Even labs that do have digital PCR technology might find it too onerous for ESR1 monitoring, since a large number of reactions would be needed to cover the various mutations required for testing.
Next-generation sequencing (NGS) platforms may also meet the sensitivity requirement, but only with specialized workflows and at certain sample inputs. Furthermore, running a single-gene test for a single sample with NGS technology is likely cost-prohibitive. Laboratories with sufficient testing volume could use NGS for ESR1 mutation monitoring, but even labs at the largest hospitals are unlikely to have such volume.
A Combination Model Using ctDNA and RNA Molecules
A new approach to ESR1 mutation testing might allow clinical laboratories to overcome these challenges and implement mutation monitoring in-house. It goes beyond the low volumes of ctDNA, taking advantage of the more abundant exosomes found in plasma to achieve the necessary sensitivity. By pairing traditional ctDNA analysis with exosomes shed by tumor cells, the entire workflow can be performed using the widely available qPCR technology that many clinical laboratories adopted for COVID-19 testing during the pandemic. With the precipitous decline of COVID-19 testing, clinical lab teams now have the opportunity to expand their test menus, and ESR1 mutation monitoring could slot in nicely on established qPCR platforms.
Researchers have already presented data about this approach at scientific conferences. A single qPCR workflow uses primers targeting allele-specific ESR1 for about a dozen relevant mutations; signal can come from ctDNA or from RNA molecules contained in exosomes. The combination of both sources makes it possible to reach the level of sensitivity needed for ESR1 monitoring. At a poster presented at the annual meeting of the Association for Molecular Pathology in 2023, scientists showed that they had been able to detect as few as three ESR1 mutations in a background of 5,000 wild type copies, for a minor allele fraction of 0.06%7.
Looking Ahead: The Future of ESR1 Mutation Monitoring
As the need for frequent ESR1 mutation monitoring increases within the HR+ metastatic breast cancer patient population, clinical laboratory teams must find new ways to meet rising demand. Send-out tests are not ideal due to their cost and turnaround time, while in-house testing with NGS or digital PCR platforms is not realistic for most labs.
An alternative approach allows clinical labs to deploy readily available qPCR technology, boosting its sensitivity by performing targeted ESR1 analysis in both ctDNA and exosomal RNA. Research has already demonstrated the sensitivity of this technique, and supports broad implementation within clinical laboratories looking to expand into ESR1 mutation monitoring.
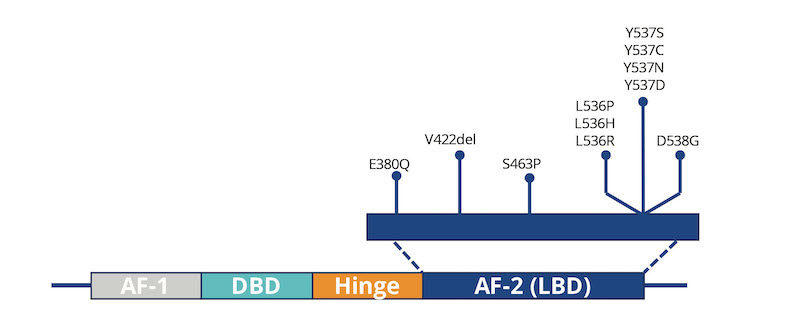
Featured Image: In patients with HR+ metastatic breast cancer, the development of ESR1 mutations can lead to resistance to standard therapies. That is especially true for mutations in the ligand-binding domain (LBD), such as those shown here. Image: Asuragen, a Bio-Techne brand.
About the Authors

John McNamara is the senior strategic marketing manager for Bio-Techne’s molecular diagnostics division. His previous life sciences and diagnostics experience includes management consulting, global product management, and marketing communications.

Tom Bittick serves as senior product manager for oncology in the molecular diagnostics division at Asuragen, a Bio-Techne brand. He has 30 years of experience in the life sciences, with prior positions at Sigma-Aldrich, Ambion, and Thermo Fisher.
References
- Hermida-Prado F, Jeselsohn R. The ESR1 mutations: from bedside to bench to bedside. Cancer Res. 2021;81:537–8.
- National Library of Medicine. PAlbociclib and Circulating Tumor DNA for ESR1 Mutation Detection (PADA-1). Available at https://clinicaltrials.gov/study/NCT03079011. Accessed February 15, 2024.
- Bidard FC, Hardy-Bessard AC, Dalenc F, Bachelot T, et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022 Nov;23(11):1367-1377. doi: 10.1016/S1470-2045(22)00555-1. Epub 2022 Sep 29. PMID: 36183733.
- Helwick, Caroline. PADA-1 Trial: With Early Identification of ESR1 Mutation, Switch to Fulvestrant in Metastatic Breast Cancer. October 10, 2022. The ASCO Post.
- National Library of Medicine. Phase III Study to Assess AZD9833+ CDK4/6 Inhibitor in HR+/HER2-MBC With Detectable ESR1m Before Progression (SERENA-6) (SERENA-6). Available at https://www.clinicaltrials.gov/study/NCT04964934. Accessed February 15, 2024.
- AstraZeneca. Camizestrant significantly delayed disease progression in advanced ER-positive breast cancer, adding at least 3.5 months benefit versus Faslodex. 8 December 2022. Available at https://www.astrazeneca.com/media-centre/press-releases/2022/camizestrant-significantly-delayed-disease-progression-in-advanced-er-positive-breast-cancer.html. Accessed February 15, 2024.
- Thibert, Julie R, et al. Development of a Novel Exosome-based, Multiplexed RT-qPCR Technology for Rapid and Accurate Detection of Circulating Tumor Acquired Resistance Variants in ESR1 at ≤ 0.1% Frequency. Poster presented at the annual meeting of the Association for Molecular Pathology. November 14-18, 2023. Salt Lake City, Utah.