Summary
Researchers developed an ultrasensitive urine test using ELISA to detect HPV16 E7 oncoproteins, offering a noninvasive method for cervical cancer screening.
Takeaways
- Noninvasive Screening: The new urine test provides a less invasive alternative to traditional Pap smears and HPV DNA tests, encouraging more women to participate in regular cervical cancer screening.
- High Sensitivity: The ELISA test demonstrated high sensitivity in detecting E7 oncoproteins in urine, correlating with different stages of cervical intraepithelial neoplasia (CIN), which are precursors to cervical cancer.
- Global Health Impact: This innovative approach could significantly reduce cervical cancer rates, especially in low- and middle-income countries, by facilitating early detection and treatment.
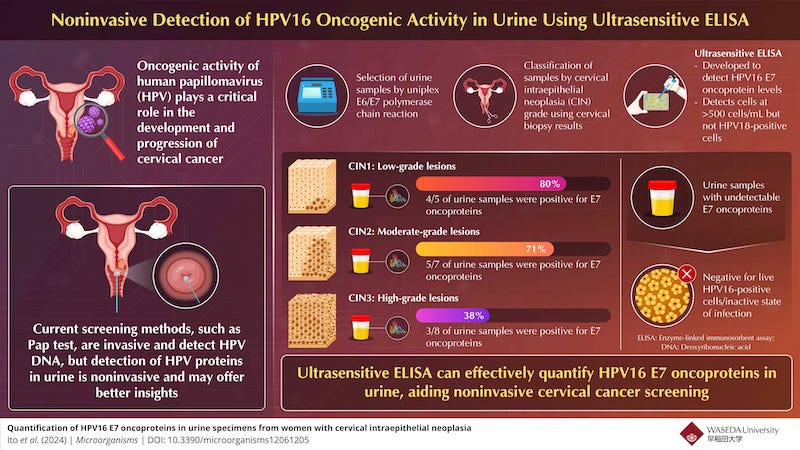
Cervical cancer is one of the most common cancers in women, with approximately 660,000 new cases and 350,000 deaths worldwide in 2022 alone. Almost all cases are linked to high-risk human papillomavirus (HPV) infections. Current screening methods involve detecting HPV DNA, but emerging research suggests that measuring the cancer-causing activity of HPV, may provide a more accurate assessment of cancer risk.
Can a new, super-sensitive test accurately measure proteins linked to HPV in urine to help detect cervical cancer?
Urine Test Shows High Sensitivity
A group of researchers led by Etsuro Ito, PhD, from the Department of Biology, Waseda University, Japan, along with Toshiyuki Sasagawa, PhD, from Kanazawa Medical University, Japan, and Martin Müller, PhD, from the German Cancer Research Center, Germany investigated to develop an ultrasensitive enzyme-linked immunosorbent assay (ELISA) to detect high-risk HPV16 E7 oncoproteins in urine. Their findings were published in Microorganisms journal.
“Cancer can be prevented by vaccination before it develops and by regular screening. But screening is a big hurdle for young women,” says Ito “Our new urine test can detect HPV16 E7 proteins, which are critical markers of cervical cancer risk, at extremely low levels. This means that women may be able to screen for cervical cancer without the discomfort and inconvenience of a traditional Pap test.”
Current Cervical Cancer Screening Methods Are Invasive
Current screening methods for cervical cancer typically involve a Pap smear or an HPV DNA test, both of which require a visit to a healthcare provider and can be uncomfortable for many women. This new urine test offers a noninvasive alternative, which could encourage more women to participate in regular screening.
The researchers used ELISA to detect the E7 oncoproteins in urine samples. The test was able to identify these proteins in the urine of women with different stages of cervical intraepithelial neoplasia (CIN), a precursor to cervical cancer. The ELISA test detected E7 proteins in 80% of women with CIN1, 71% with CIN2, and 38% with CIN3, suggesting that the presence of E7 oncoproteins correlates with lower-grade CIN lesions. The researchers theorize that this discrepancy may be due to variations in the HPV life cycle or oncogenic activity.
“We believe that the E7 oncoprotein is critical in the early stages of HPV-related cervical carcinogenesis and E7 may play a more significant role in the progression of CIN1 and CIN2 than in CIN3,” says Ito.
Urine Test Aligns with Goal to Reduce Cervical Cancer Rates
This innovative approach aligns with global health goals to reduce cervical cancer rates, especially in low- and middle-income countries where access to traditional screening methods is limited. With further development and validation, this urine test could become a standard tool in the fight against cervical cancer, helping to save lives through earlier detection and treatment. The development of a noninvasive urine test for detecting HPV-related proteins represents a significant step forward in cervical cancer screening. It offers a promising solution to increase screening rates and reduce the incidence of cervical cancer worldwide.
“This new method holds great promise for early detection and prevention of cervical cancer. We are optimistic that further development and validation of this assay will lead to its widespread use in clinical settings,” says Ito.
Featured image: Researchers have now validated a noninvasive method for screening cervical cancer. This could open new avenues for cancer screening by collection of urine sample by the patients themselves and then delivering it to medical facility for testing. This alternative method is a step forward to eradicate cervical cancer by lowering the barriers related to screening. Image: Dr. Etsuro Ito from Waseda University, Japan