New platforms and newer ideas are reshaping the future of clinical lab testing
By Steve Halasey and Jenny Lower
Technologies rarely advance at a steady and predictable pace. Often, in fact, they seem to lurch along unsteadily, buffeted by the vagaries of investor interest, competing intellectual properties, regulatory hurdles, and marketplace challenges. And the more complex the technology, the greater the number of difficulties needing to be overcome.
That seems to be especially true in the realms of in vitro diagnostics and clinical laboratory testing, where cutting-edge technologies often require years of hard work and patience before they’re ready for the marketplace—and vice versa.
The past year has been anything but typical in this regard, with FDA providing market clearance for diagnostic applications of two major platforms: MALDI-TOF mass spectrometry and high-throughput genomic sequencing. Without question, the value of these platforms will become even greater in the future as they are refined through adoption and use.
But make no mistake, bright minds are already laying the groundwork for the next wave of advances in clinical testing. In this issue, CLP visits with just a handful of companies that are working to bring new diagnostic technologies to market and improve patient care. For more about all of the products discussed here, see the extended versions linked to each listing below.
Illumina Brings in the Business
Since last November, when FDA cleared Illumina’s MiSeqDx high-throughput sequencer for diagnostic applications, the potential of next-generation sequencing has captured the imagination of many scientists and laboratorians—and with it their engagement in developing clinical tests based on Illumina’s platforms.
In February, Illumina took further steps to stimulate the adoption of NGS systems by launching the Illumina Accelerator Program. Billed as the world’s first business accelerator focused solely on creating an innovation ecosystem for the genomics industry, the program’s goal is to speed the time to market and lower the barriers to entry for entrepreneurs, start-ups, and early-stage companies working on scientifically and commercially promising NGS applications.
Through the accelerator’s 6-month program, Illumina will provide invited participants with technology and business guidance and $100,000 in support, including access to sequencing systems and reagents, as well as fully operational lab space in close proximity to the company’s planned R&D facilities in San Francisco’s Mission Bay.
Through a competitive process, the Illumina Accelerator Program will select up to three companies to participate in each 6-month session per year.
See the extended version of this story here.
bioMérieux Gets First Mover MS Advantage

The Vitek MS by bioMérieux is the first
clinical mass spectrometry system to receive FDA clearance for the US market.
From its headquarters in Marcy l’Etoile, France, diagnostics giant bioMérieux had more than one reason to celebrate last year. Not only did the company mark its 50th anniversary, it also gained first-to-clinical-market advantage with the launch of its new diagnostic platform, dubbed Vitek MS.
Vitek MS is a mass spectrometry system intended for the identification of pathogenic organisms in clinical laboratory applications. FDA granted 510(k) de novo clearance for the system in August 2013, making it the first clinical mass spectrometry system to be cleared for the US market.
Speed, accuracy, and breadth are all hallmarks of the new system. The Vitek MS can perform up to 192 different tests in a single automated series of testing, with each test taking about 1 minute. The system performs well when compared with nucleic acid sequencing, achieving comparative accuracy of 93.6%.
By automatically running the spectra through a library of organisms owned by bioMérieux, the Vitek MS is able to identify the microorganisms of the sample very precisely. The Vitek MS library represents the vast majority of bacterial and fungal infections that afflict humans. As a world leader in clinical microbiology, bioMérieux holds the largest private collection of microorganism strains in the world.
See the extended version of this story here.
MedMira Multiplexes Rapid POC Testing
Faced with decreasing budgets and constrained resources, healthcare providers are seeking high-quality diagnostics that deliver efficiency in every way, from performance to results. With the unique capability of delivering multiplexed results in a rapid test format requiring just one drop of specimen, MedMira is developing a new class of diagnostic testing solutions for its customers.
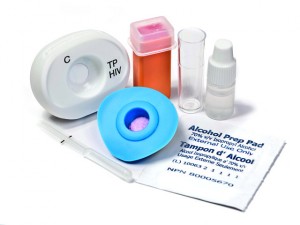
MedMira’s Multiplo TP/HIV test is a multiplexed point-of-care test for the simultaneous detection of antibodies to syphilis and human immunodeficiency virus (1 and 2).
Headquartered in Halifax, Nova Scotia, Canada, MedMira is developing a robust pipeline of tests based on its patented Rapid Vertical Flow (RVF) technology. The technology facilitates the formation of highly specific antigen-antibody reactions, allowing specific biomarkers in many sample types—including human whole blood, serum, or plasma—to be captured and visualized on a unique membrane.
Under its Multiplo brand name, MedMira is currently working on two multiplexed tests. The first is a combined HIV and syphilis test, primarily for the neonatal market, which is already in use in several Latin American countries. The second is a combined HIV, hepatitis B, and hepatitis C test, which has received approval for use in some countries. This product is currently going through FDA premarket review for entry to the US market.
See the extended version of this story here.
Menssana Research Breath Test for Disease Biomarkers
Menssana Research is the leading developer of advanced breath tests for the detection of disease. Early detection of diseases often offers significant benefits: Some cancers can be more easily treated if they are detected early and treated promptly.

Menssana’s BreathLink system collects, concentrates, and analyzes a breath sample, and then uploads the findings to the company’s New Jersey-based lab.
Michael Phillips, MD, founded Menssana Research after speculating whether diseases could be detected in their earliest and most treatable stages simply by analyzing breath. Perhaps the least invasive of diagnostic tests, breath testing offers the advantage of accessibility: Even the very elderly and the very ill can generally donate a breath sample without inconvenience. But chemical analysis of breath remains technically difficult, keeping breath testing to a minor role in medical diagnosis until recently.
Technical advances at the Menssana Research laboratories have established a new role for breath testing in the early detection of disease. Scientists there have developed a portable breath collection apparatus (BCA) that can collect breath samples for highly sensitive laboratory analysis virtually anywhere. The BCA has been adopted for clinical research studies in hospitals in the United States and Europe.
See the extended version of this story here.
Thermo Fisher–Harvard Collaboration Targets Major Advances in Proteomics Quantitation
Scientists working to discover protein-based biomedical breakthroughs are constrained by the time and cost required to identify and quantify large numbers of proteins. Thermo Fisher Scientific and researchers at Harvard Medical School have teamed up to develop promising new ways to perform protein quantitation on a much larger scale than currently possible, with the goal of accelerating the discovery of effective therapies.

Steven Gygi, PhD, of the Thermo Fisher Scientific Center for Multiplexed Proteomics at Harvard Medical School.
The two organizations have established the Thermo Fisher Scientific Center for Multiplexed Proteomics at Harvard Medical School. The new facility is headed by Harvard professor of cell biology Steven Gygi, PhD.
The work of the center focuses on advancing multiplexing technology—the ability to analyze multiple protein samples in a single mass spectrometer run—in order to gain new insights into the mechanisms of life. Gygi has pioneered the use of “tandem mass tags” (TMTs) for multiplexed protein analysis, and researchers will combine this expertise with new-generation mass spectrometry technology to seek ways of increasing the amount of quantitation that can be performed by orders of magnitude, without sacrificing data quality.
See the extended version of this story here.
MALDI-TOF Makes Inroads into Clinical Labs for Bacterial Identification
The potential for MALDI-TOF mass spectrometry to produce bacterial identifications that are better, faster, and cost less to produce has been well documented in the scientific literature. Among the many peer-reviewed articles on microbiological applications of MALDI-TOF MS, more than 300 have been completed using the Bruker MALDI Biotyper, first launched in 2007.
In November 2013, Bruker was granted FDA clearance to market its MALDI Biotyper CA system in the United States for the identification of gram-negative bacterial colonies cultured from human specimens.
Further applications of the MALDI Biotyper CA system are being developed, both internally and with collaboration partners. For example, Bruker is working on antibiotic resistance mechanism detection. While culture-based methods are still the gold standard for antibiotic resistance testing, the first steps toward the use of MALDI-TOF MS for rapid detection of antibiotic and antimycotic substances have been very promising.
See the extended version of this story here.
Paper Chase
Researchers at the University of Washington are working to develop a handheld, paper-based device that could detect infectious diseases using samples collected and tested at the point of care. Requiring no complex instrumentation, the multiplexable and disposable autonomous nucleic acid amplification test system has initial targets of drug-resistant Staphylococcus aureus and influenza. The technology could hold promise for pathogen identification in remote areas, as well as densely populated areas such as hospitals, prisons, and military bases.

Paul Yager, PhD, displays an early prototype of the paper-based molecular diagnostic under development at the University of Washington.
Led by Paul Yager, PhD, former chair of bioengineering at the University of Washington, the team comprises UW research assistant professor Barry Lutz, PhD; UW biochemistry professor David Baker, PhD; Oregon State University senior research assistant professor Elain Fu, PhD; and researchers from Seattle Children’s Hospital, Epoch Biosciences, PATH, and GE Global Research. Known by Yager and his colleagues as a “two-dimensional paper network,” or 2DPN, the test relies on nucleic acid amplification that activates when the substrate is exposed to specimens via a nasal swab. In less than an hour, a pattern of colored dots will appear to indicate the presence of certain pathogens. Users can then photograph and transmit the results via cell phone, while a medical professional or software at the other end interprets the pattern and issues a therapy recommendation.
See the extended version of this story here.
Electrozyme Decentralizes the Clinical Lab with Printed Biosensors
Clinical laboratories currently rely on expensive instrumentation to conduct the full range of diagnostic work-ups. Despite the increasing drive toward automation, most diagnostics performed in clinical laboratory settings are inherently slow, prone to error, and can only be operated by extensively trained personnel.

Cofounders Joshua Windmiller, PhD, (left) and Jared Tangney, PhD, display Electrozyme’s microelectronic sensor backbone and printed electrochemical sensor sheet, respectively.
To alleviate many such challenges faced in clinical laboratories, Electrozyme is endeavoring to become the world leader in the development of printed electrochemical biosensors. One of the company’s high-profile offerings includes a temporary tattoo that adheres to the skin and tracks lactate, a form of lactic acid emitted in sweat. The sensor captures physiological information without blood testing and transmits the data in real time to a mobile device, helping athletes ensure proper hydration and maximize efficiency throughout their workouts. While the company has thus far focused on the sports, fitness, and wellness domains, CEO Joshua Windmiller, PhD, hopes this strategy will enable Electrozyme to build revenue in nonregulated applications and invest in developing a scalable clinical model.
See the extended version of this story here.