A familiar refrain for those who have traveled on London’s tube, “mind the gap” is a warning to take caution while crossing between the station platform and the train door. In oncology, patients and clinicians have been minding their own gap — the precision-medicine gap — for decades.
By Jarret Glasscock, PhD
For decades, travelers on the London Underground have heard the brief but impactful phrase “Mind the Gap” broadcast over a loudspeaker as the train approaches and passengers enter or exit the cars. The gap — a physical distance between the curved tracks and the platform — was deemed unsafe but unavoidable due to challenges in track and railcar engineering. In the absence of a physical device to close the gap, this auditory warning has been the ever-present stop gap to ensure passengers are aware and prepared to navigate safely.
In medicine, a similar warning is ringing. This gap, known as the precision-medicine gap, represents the biggest threat to realizing truly patient-centered medicine today. Contrary to the London Underground, however, the precision-medicine gap could be closed. First and perhaps most important, is that there are indeed technologies available that can help us bridge this gap. However, we must also recognize the origin of the gap is tied to a number of factors, and it’s not solely engineering or technological advancements that are needed to create a lasting solution. Finally, it’s important to note that the gap is not static, rather it will continue to grow unless we intervene. At the heart of this intervention is a class of technologies known as predictive diagnostics.

The Gap Between Patients and Therapies
One of the most easy-to-visualize embodiments of the precision-medicine gap is in oncology. Specifically, the patient populations eligible for immunotherapy, as shown in Figure 1. This graphic represents data summarized from an analysis published in the Journal of the American Medical Association (JAMA) Network Open titled, “Estimation of the Percentage of U.S. Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs.” This class of treatments have revolutionized our expectations for treatment success in oncology. For patients who respond, the impact is lasting and transformative. As such, the manufacturers of these therapies continue to invest in clinical studies that expand the indications and stages of disease where patients are eligible to receive these treatments. This is shown by the growing eligible patient population (as a percentage of total cancer diagnoses), represented by the grey bars.
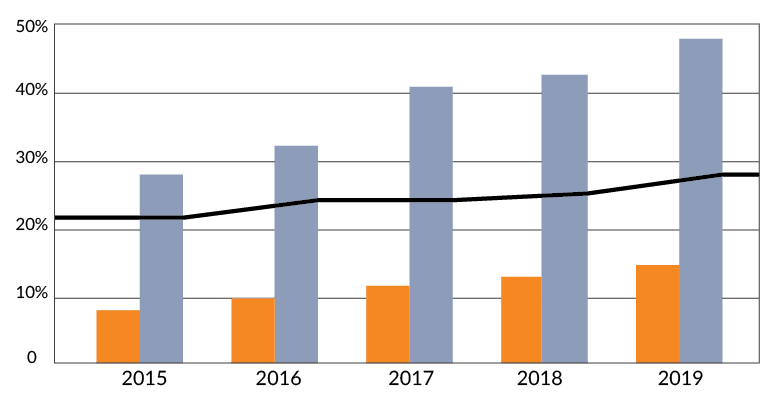
However, the percentage of patients who respond to these therapies continues to disappoint. On average across indications, this percentage is less than 25% of eligible patients, also shown in the orange bars of Figure 1. And so, the gap between these patient populations widens, depicted by the bold black line of Figure 1, which continues in an upward trend.
Technology’s Role in Closing the Gap
As alluded to earlier, there is a technological solution that can enable us to close this gap. The category of predictive diagnostics, which is driven by advancements in molecular characterization, can enable us to better match patients with the therapies that will benefit them most. So, rather than treating a large patient population and seeing less-than-ideal response rates, with these diagnostic tools we can predict, ahead of treatment, who will respond and prioritize those would-be responders for treatment with that particular therapy. Achieving stratified medicine through more precise predictive diagnostics results in multi-fold increases in predictive accuracy and response rates that are more compelling for patients and their treating physicians. Beyond improved response rates, these technologies can also help clinicians minimize adverse events, avoid unwanted treatment side effects (monotherapy responders avoid chemotherapy combinations), and create more high-value healthcare. With oncology costs expected to rise by 9%–12% annually through 2023, predictive diagnostics represent an important facet of value-based healthcare. As summarized by the team at Aptitude Health, “Payors and patients alike are anxious to control costs….Even as new and better therapeutic approaches emerge, pharma companies can expect to face mounting pressure to reduce treatment costs.”2 Innovation in therapy alone will not help us close the precision-medicine gap.
Gap Between Investment in Therapies and Diagnostics
And yet, historically, investment in oncology has focused on basic science research, causes of cancer, and especially for pharmaceutical companies: treatments. When we look at the funding areas of the American Cancer Society3 as a case study, we see this prominently featured. Funding for diagnostics represents a fraction of the funding total, more so when compared to the top three areas noted.
American Cancer Society Publicly Disclosed Funding*:
Biology = 36.8%
Cause/Etiology = 12.8%
Treatment = 16.5%
compared to:
Early Detection, Diagnosis, and Prognosis = 7.1%
This dynamic between therapies and diagnostics is complex. While therapy development and clinical trials are extraordinarily expensive, the use of biomarkers and diagnostics tests often enable more efficient and less costly clinical trials, streamlining enrollment and requiring less patients. But that may come at a cost at the time of commercial launch, with a narrower market or smaller patient population eligible for treatment. In one publication, this was described as the “bottom-line effect” — because biological markers may identify smaller patient populations who respond, the resulting diagnostics may “limit payoffs.”4 Scientifically and ethically, the reality is that the partnership between diagnostics and therapies is an essential part of closing the precision-medicine gap. The funding disparity between treatments and diagnostics, paired with a profit-over-patient mindset both demonstrate an opportunity for improvement to help us close the gap. A biomarker and diagnostic-led approach to treatment decisions lends itself well to the value-based healthcare initiatives already underway in many institutions.
Gap Due to Racial and Economic Disparities
Last but certainly not of least importance, as described in a similarly-titled and excellent article by a team at MD Anderson (“Mind the Gap: Precision Oncology and Its Potential to Widen Disparities”), the pursuit of more precise medicine is both impacted by and has the potential to exacerbate existing disparities resulting from racial and economic barriers.5 When we don’t consider a diverse patient population in clinical studies, the resulting treatments and diagnostic tools have limited impact.
Both when building and validating therapies and diagnostic technologies, we need to recruit diverse clinical trial participants. This includes considering standard demographics such as age and race, but also less-often considered demographics such as ethnicity, geography, and socioeconomic status. This can be accomplished through decentralized clinical trials, which enable patients to join either through a central site (large healthcare center) or as an individual, through direct-to-patient recruitment.
Clinical research partners, such as Curebase, have developed software and support tools to enable such decentralized, virtual trials. It’s imperative we leverage these tools when building both therapies, and the diagnostic tools we’ve described as key to closing the precision-medicine gap. And, as described by the team at MD Anderson, once these therapies and tools are available, consciously advocating for reimbursement across both public and private payers allows more equitable access for patients.
Bridging the Gap
I find it interesting that as we, as an industry, have been in pursuit of precision medicine, it is not medicine at all that will close the gap but a number of other important factors. These factors include diagnostic technologies, funding, and patient diversity. The fact that our industry’s yearly precision-medicine conferences have evolved over recent years to not only highlight clinical trials of new drugs but also presentations, roundtable discussions, and entire sessions focused on these three areas bodes well for putting the pieces in place to bridge this gap.
For many, the discussions around these three topics are considered ancillary to the primary discussion around drug targets, drug development, and clinical trials. With the more than a 500% increase in clinical trials in the last 5 years, and much of that growth being attributed to precision medicines (i.e., drugs that work in a subset of the patient population), these areas will not only continue to become larger considerations, but will be strategic competitive advantages for companies to excel where others not considering them will fall short in comparison. Moving from “minding the gap” in precision medicine to closing the gap in precision medicine is requiring diverse and unexpected considerations beyond what were discussed back when the terms and concepts of precision medicine and personalized medicine were first introduced.
Precision medicine is a continuum and I look forward to us continuing to move the industry towards more impactful patient care in the years to come, not only through innovative therapies but with innovations and changes in predictive diagnostics, use of resources, and more thoughtful trial design.
*NOTE: Total funding includes other areas such as Cancer Control, Survivorship (including quality of life and end of life), Outcomes Research, and uncharacterized research.
ABOUT THE AUTHOR
Jarret Glasscock, PhD, is the co-founder and CEO of Cofactor Genomics. After contributing to the Human Genome Project and sequencing the first Cancer Genome while faculty at Washington University, he founded Cofactor Genomics to leverage the power of RNA to diagnose disease. Jarret’s background in genomics and his passion for precision medicine has enabled Cofactor to champion the creation of a new category of diagnostics, built on RNA models, that are changing cancer treatment decisions.
REFERENCES
- Haslam A, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw Open. 2019;2(5):e192535. doi:10.1001/jamanetworkopen.2019.2535
- Emerging Oncology Trends: 2021 and Beyond. Aptitude Health. January 8, 2021. https://www.aptitudehealth.com/blog/emerging-oncology-trends-2021/
- Current Investment by Areas of Research. American Cancer Society. May 2021. https://www.cancer.org/research/currently-funded-cancer-research/investment-by-research-areas.html
- Ten Trends Transforming the Business of Oncology. OBR Oncology. September 2011. https://www.obroncology.com/blog/ten-trends-transforming-the-business-of-oncology-2
- Ryan W. Huey, Ernest Hawk, and Anaeze C. Offodile II. Mind the Gap: Precision Oncology and Its Potential to Widen Disparities. Journal of Oncology Practice 2019 15:6, 301-304