
The DCA Vantage™ system from Siemens Healthcare Diagnostics better manages diabetes patients by enabling quick results and actionable physician-patient conversations at the time of the visit.
Point-of-care testing (POCT) technology provides immediate, portable, and convenient medical diagnostics near the site of patient care. POCT’s near-instantaneous results allow health care providers to make treatment decisions for their patients in a shorter amount of time compared to traditional laboratory diagnostics. Faster test results translate to rapid treatment for patients—which can save lives in critical care scenarios.
POCT: THEN AND NOW
POCT has been around since the 1980s. Early forms of the technology were mainly used for collecting data, such as measuring blood glucose levels. The clinical and operational benefits of POCT were first evident in the operating room. For the management of cardiovascular surgery patients, POCT technology has been an indispensable aid. The technology has advanced to not only coordinate with financial and reimbursement protocols and inform physician-patient communication, but also to integrate with electronic medical records (EMRs) and wireless and Web technologies.
POCT technology is now used in primary care, emergency department, and intensive care environments. Typical assays include blood glucose testing, blood gas and electrolyte analysis, coagulation testing, cardiac marker diagnostics, drug screening, urine dipsticks, pregnancy testing, fecal occult blood analysis, food pathogen screening, hemoglobin diagnostics, infectious disease testing, and cholesterol screening.1 The most common POCTs used in US hospitals include glucose (99%), coagulation (62%), blood gas (50%), chemistry (36%), hematology (28%), urinalysis (15%), and cardiac (3%) diagnostics.2 In short, POC technology is almost ubiquitous.
POCT systems for detecting pathogens are often easy-to-use, membrane-based test strips enclosed in plastic. Systems for rheumatology diagnostics typically require only a small amount of sample (one drop of blood, rather than an entire vacuutainer), which means no transportation, delay, or preprocessing of the specimen. Thus, POCT minimizes blood drawing from the patient, which makes this process particularly accurate in pediatric and neonatology wards. The lack of sample processing translates to institutional savings.
Benefits of using POCT include rapid decision-making and reduced operating times, postoperative care time, emergency department time, number of outpatient clinic visits, and hospital beds required, ensuring optimal use of health care professional time.
POCT technology may sometimes fall short in accuracy, adaptability to older data management systems, time required to train personnel to use the technology, and high dependence on informational technology support. The following sections address these issues and discuss the potential future of POCT technology.
PERCEIVED POCT BENEFITS
Streamlining Communication
Using POCT streamlines communication between physician and patient, allowing treatment to be started sooner. “I feel that this is the primary advantage of POC testing,” says Andrew Schaeffer, senior R&D scientist, Quantimetrix Corp, Redondo Beach, Calif. “The obvious example is use in the hospital emergency room, where decreased disposition time can be expected to lead directly to improved outcome. Hence, the explosion in the use of POC-type cardiac marker testing.”
Outcomes can also be improved. “The fundamental value proposition of POC testing is the streamlining of communication and the enabling of improved workflow, patient care, and outcomes,” says David Stein, PhD, CEO, Point of Care Business Unit, Siemens Healthcare Diagnostics, Deerfield, Ill. “This includes both hospital scenarios where an improvement in the turnaround time for critical care tests such as blood gas and cardiac markers result in faster treatment, enabling better outcomes.
“An excellent example in the physician’s office is when HbA1c testing is performed for monitoring diabetes,” Stein adds. “Since the test results are available during the patient visit, the physician and patient can discuss them immediately, as well as any necessary changes that the physician recommends for the patient’s treatment plan.”
Ease and Speed of Use
POCT is easier to use and provides faster results than many other diagnostic technologies used in the lab. The very nature of the technology suggests speed, as testing is done closer to the patient and sample transportation time is eliminated. POCT is very easy to use, as evidenced by many of the tests being CLIA-waived. Speed and ease of use are particularly beneficial in settings such as emergency departments or operating rooms, or the intensive care unit where seconds count and physicians need immediate test results to help inform critical treatment decisions.
Freeing Up Time in the Central Lab for STAT Testing
Removing the burden of simple tests from central lab personnel should result in more rapid STAT testing. Allowing the central lab to concentrate on more complex tests may also lead to higher revenues.
PERCEIVED POCT CONCERNS
Interrupting the Central Lab’s Revenue Stream
Increased reliance on POCT will reduce the amount of work sent to the central lab. For many labs, this will spur economic dilemmas. However, this problem may be partly offset by the documented shortage of central medical lab technicians in the workforce. In 2003, 50% of labs had difficulty in recruiting and hiring clinical lab workers; 91% had difficulty in hiring for at least one shift; night shifts were most difficult to fill (82%) for medical technologist positions; and evening shifts were most difficult to fill (72%) for medical lab technologist positions.3 And POCT can free up the central lab to perform more complicated, and more expensive, diagnostics.
Less Accurate than Central Laboratory Tests
Some say the emphasis POCT places on portability and simplicity has resulted in a cost in terms of accuracy in complex tests (eg, not urine dipsticks). The struggle to establish the effectiveness of handheld glucose meters in hospitals would seem to back this up. A 2010 article in Clinical Chemistry by Lenters-Westra found that six of eight commercially available POC HbA1c instruments failed to meet Clinical and Laboratory Standards Institute (CLSI) criteria.4 As time passes, however, instrument manufacturers will continue to close the performance gap between lab and POC instruments.
Generalizations may be fruitless; tests’ effectiveness needs to be weighted on a case-by-case basis. “When comparing the accuracy of POCT with central lab tests, it really depends on the actual test, method, and analyzer being used,” says Siemens’ Stein, who heads the company’s Point of Care Business Unit. “There are numerous examples where a POC test is as accurate as its central lab contemporaries.”
USE OF THE POCT IN DECENTRALIZED HEALTH CARE
Stein says decentralization will continue to occur, which not only enhances productivity, but also improves quality of care for patients and health care professionals, and improves patient convenience by reducing the number of required patient visits. “This is particularly important for critical conditions where every minute delay in treatment initiation has a negative effect on patient outcomes, and in patient populations such as the elderly, who often find it challenging to arrange the physical assistance and transportation necessary to [cap off] a physician or hospital visit with a follow-up visit to an in vitro diagnostic testing facility,” he says. Clearly, the more decentralized an operation, the more it will benefit from POC applications.
Debate rages over a reduction in overall health care costs as a result of health care decentralization. A study by Laurence et al in BMC Health Services Research in 2010 found that cost savings were variable, at best.5 The study concluded that the primary benefit of POCT is found in improved patient satisfaction and adherence to therapeutic regimens.
POCT REGULATIONS
POCT is regulated by several agencies. It appears that the CLSI and ISO 15197 guidelines for glucose meters will be tightened to 15% allowable error from the current 20%. CLSI also released guideline POCT08-A in December 2010, which is designed to regulate noninstrument POC devices such as urine dipsticks.
Institutions may struggle to meet the new standards of use put in place by the Centers for Medicare and Medicaid Services (CMS). Originally, CMS provided six elements for demonstration of competency, allowing sites to be flexible in selecting which of the six elements were relevant to their environment. Now, CMS is requiring all six elements of competency for each operator.6 The requirements for testing specimens of known concentrations, direct observation during training on POCT technology, and finding the time to perform quality controls may prove a struggle for some institutions.
REIMBURSEMENT
Tests performed at the point of care provide beneficial extensions to lab services. Numerous studies attest to the cost for providing this service. Organizations face many challenges in POCT billing, including the ability to accurately capture information and familiarity with regulatory and payor guidelines. Billing for POCT can generate revenue to offset some or all of the expenses for the service.7
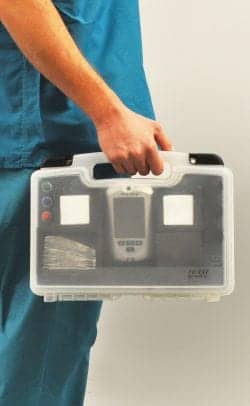
The StatStrip Wireless Tote from Nova Biomedical offers bidirectional wireless connectivity for StatStrip hospital glucose meters.
POCT IN DEVELOPMENT
Noninvasive POCT
The possibility of making noninvasive transcutaneous POCT analysis is extremely attractive, particularly in neonatology and critical care settings. The use of percutaneous bilirubin meters in neonates has become more popular in recent years. However, studies from the Mayo Clinic, Rochester, Minn, suggest these devices can overestimate serum bilirubin levels when compared to lab determinations using the Doumas reference method.8 These devices have only been approved for use in limited populations. Performance in other ethnicities with different skin tones has not been well-characterized.
Nanotechnology and POCT
The growing field of nanotechnology is opening up new opportunities in POC, specifically with sensor design such as sensor-telemetry integration, wound-monitoring, e-nose, neural stimulation, cardiac mapping, implantable sensors, and immobilization of bio-selective proteins; quantum dots; extremely sensitive multiplex tests for analyte detection; and displacement assay technology modeled for optimization of key nanotechnology components.
For example, low-volume whole-blood sensors can provide accurate blood analysis for people experiencing myocardial infarction or other life-threatening cardiac events. POCT can almost instantaneously analyze blood enzymes and clot time to determine the proper life-saving treatment. The sensor system under development at the University of Ulster, Londonderry, Ireland, and the Indian Institute of Technology, Bombay, India, will use carbon nanotubes to filter out blood cells, which will prevent them from adhering to the sensor or distorting the result.9
Quantum dots, one of the most popular nanotechnology tools, could be used to identify cancer by its molecular expression profile to best classify personalized treatment.10 Initially, integrating quantum dots with lateral flow devices could mean serious strides in cancer diagnostics that in time may increase uses of POCT.
Integrating POCT into EMRS and Other Data-Collection Applications
The Connectivity Industry Consortium developed the POCT1-A standard in 2001 (updated in 2006). The goal of the Consortium was to provide a set of interface standards for manufacturers that would allow users to integrate data seamlessly between hospital information systems and POCT devices. Unfortunately, the standard only provides a framework for connectivity. The actual application of the standard is not enforced with manufacturers. This has resulted in varying degrees of adoption—which raises the question: Does this standard have real value for the industry? Despite the lack of widespread adoption, David E. Sterry, MT(ASCP), CLSI director of standards, says he has recently seen an increase in interest in POCT1-A.
Major benefits are obtained when the output of a POCT device is made available immediately within an EMR. Results can be shared instantaneously with all members of a patient’s medical team, enhancing communication by decreasing turnaround time.
With the increase of wireless POCT technology, network downtime will not be tolerated. Health care institutions must ensure that download stations for POCT devices are always functioning, servers must always be on the network, and connections to host systems must never be dropped. This often translates into a significant increase in IT resources and costs.
Abbott Point of Care Inc, Princeton, NJ, has received clearance from the FDA to begin marketing the i-STAT® 1 Wireless handheld, a new wireless version of the i-STAT POCT system that is widely used in hospitals, emergency departments, and physician’s offices. The i-STAT 1 Wireless handheld will allow real-time transmission of diagnostic test results generated by i-STAT 1 directly from the patient’s bedside.11
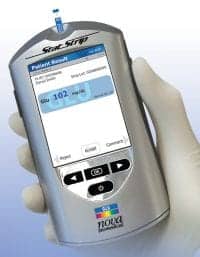
i-STAT® 1 Wireless Handheld
Abbott’s handheld device can potentially save precious time by allowing caregivers to perform critical tests at the bedside and then transmit test results immediately so they can be reviewed by physicians anywhere they have access to EMRs. Eliminating back-and-forth movement from the bedside to a desktop terminal gives staff members the ability to better focus on the patient.
With the 10-minute i-STAT cardiac troponin test cartridge, for example, emergency department staff can test, transmit, and treat from the bedside. This helps health care providers maintain compliance with testing guidelines that call for a turnaround time of less than 60 minutes. By sharing these test results via an existing wireless network, caregivers have rapid access to information that can help determine if a patient is having a heart attack.
Nova Biomedical, Waltham, Mass, recently announced the availability of a wireless connectivity solution for StatStrip® Glucose and StatSensor® Creatinine meters.12 The Nova StatStrip Wireless Tote provides bidirectional, wireless connectivity of StatStrip or StatSensor test results and patient data to or from a lab or hospital information system. StatStrip Wireless Tote provides rapid charting of test results and patient data to enable the frequent therapy decisions needed for patients on glycemic control protocols.
Other handheld glucose meters are typically placed in a docking station that is connected to a wired Internet connection in order to communicate with the network. Patient test data is not uploaded until the device is placed in the dock—sometimes occurring only at the end of a shift. Because glucose tests and therapy decisions are being made frequently on hospitalized patients, the need for continuous, secure, wireless connectivity is becoming essential.
As hospitals move to upgrade their IT infrastructure to support networked devices, many are looking to wireless technology to improve charting of POCT results, reduce the costs of hardwiring a facility, and support additional capabilities such as asset and patient tracking.
THE FUTURE OF POCT
As POCT evolves, an emphasis will be placed on instant information, increased EMR coordination, continuous glucose testing and monitoring, open IT standards, Web-based communication and connectivity, and advanced data security. Ultimately, future POCT technologies must ensure improved patient care, accuracy, cost reduction, and revenue generation.
Sarah Michaud is a contributing writer for CLP.
REFERENCES
- Point of Care Diagnostic Testing World Markets: TriMark Publications LLC; 2011.
- Enterprise Analysis Corp. In. Stamford, CT; 2001.
- Ward-Cook K, Chapman S, Tannar S. 2002 Wage and Vacancy Survey of Medical Laboratories. Lab Medicine. 2003;34:631-638.
- Lenters-Westra E, Slingerland RJ. Six of eight hemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin Chem. 2010;56:44-52.
- Laurence CO, Moss JR, Briggs NE, Beilby JJ. The cost-effectiveness of point of care testing in a general practice setting: results from a randomised controlled trial. BMC Health Serv Res. 2010;10:165.
- Laboratory General Checklist, Statement GEN.55500 and Point of Care Checklist. Northfield, IL; June 17, 2010.
- MacMillan D. Reimbursment for point-of-care testing. Point of Care. 2002;1:253-258.
- Karon BS, Wickremasinghe AC, Lo SF, Saenger AK, Cook WJ. BiliChek transcutaneous bilirubin meter overestimates serum bilirubin as measured by the Doumas reference method. Clin Biochem. 2010;43:1009-12.
- Research set to revolutionize cardiac testing. University of Ulster, October 12, 2007. Available at: news.ulster.ac.uk/releases/2007/3443.html. Accessed May 14, 2011.
- Smith AM, Dave S, Nie S, True L, Gao X. Multicolor quantum dots for molecular diagnostics of cancer. Expert Rev Mol Diagn. 2006;6:231-244.
- FDA clears Abbott’s i-STAT 1 wireless point of care testing system. Abbott, March 29, 2011. Available at: www.abbottpointofcare.com/pdfs/028706%20Rev%20A%20i-STAT%201%20Wireless%20Press%20Release.pdf. Accessed May 14, 2011.
- Wireless connectivity now available for Nova’s Hospital Meters. Nova Biomedical, 2011. Available at: www.pointofcare.net/Nova_Wireless_Press_Release.pdf. Accessed May 14, 2011.




