One-sentence summary:
Despite limited progress in prognosis over decades, recent advancements in molecular diagnostics and site-specific therapies are transforming the clinical management of cancer of unknown primary (CUP).
Three brief takeaways:
- Persistent Challenge: CUP remains a major cause of cancer-related death due to its elusive primary origin and poor prognosis despite traditional chemotherapy.
- Diagnostic Breakthroughs: Modern molecular techniques have drastically improved CUP diagnostic accuracy, enabling tailored treatments.
- Promising Therapies: Recent clinical trials, such as Fudan CUP-001 and CUP-002, highlight the effectiveness of site-specific and second-line combination therapies in improving patient outcomes.
In a study led by Dr. Zhiguo Luo, Department of Medical Oncology, Fudan University Shanghai Cancer Center, cancer of unknown primary (CUP), a set of histologically confirmed metastases that cannot be identified or traced back to its primary despite comprehensive investigations, accounts for 2%-5% of all malignancies. CUP is the fourth leading cause of cancer-related deaths worldwide, with a median overall survival (OS) of 3-16 months. CUP has long been challenging to diagnose principally due to the occult properties of primary site.
No Significant Advancements in Prognosis of Cancer of Unknown Primary
Over the past few decades, empirical chemotherapy, containing gemcitabine/platinum or taxane/platinum regimens based on the histological types of cancer of unknown primary, has been recognized as the cornerstone of therapy before site-specific first-line therapy is proposed. Despite the application of chemotherapeutic regimens in the clinical management of CUP, there has been no significant improvement in prognosis, which has planted the seeds for the proposal of site-specific therapy.
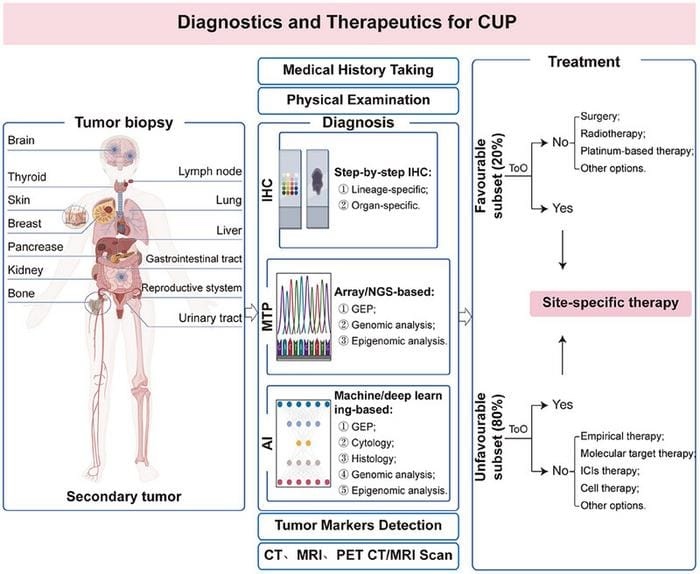
In the current era of molecular diagnostics, advancements in methodologies based on cytology, histology, gene expression profiling (GEP), and genomic and epigenomic analysis have greatly improved the diagnostic accuracy of cancer of unknown primary, surpassing 90%. Advancements in the diagnosis of CUP have laid a solid foundation for the application of site-specific therapies, ultimately improving patient outcomes for those affected by this condition.
Conducting Studies
In the last decade, several clinical trials have been conducted globally to investigate and determine the efficacy and safety of site-specific therapy compared to empirical chemotherapy for CUP. However, currently available studies comparing site-specific therapy with empiric chemotherapy exhibit significant deficiencies and notable limitations, which include study design, patient recruitment criteria, heterogeneity among the cancer of unknown primary classifiers, as well as incomparable therapies.
In 2017, Professor Xichun Hu and Zhiguo Luo designed a pioneering worldwide prospective randomized phase III study, which was known as Fudan CUP-001 (ClinicalTrials.gov, NCT03278600), to investigate the clinical efficacy and safety of 90-gene expression assay-directed site-specific therapy compared to empirical chemotherapy. Our study proved that site-specific therapy contributed to a significantly longer median PFS compared to empirical chemotherapy (9.6 months vs. 6.6 months; p=0.017). This significant breakthrough not only opens a new chapter for the clinical management of CUP, but also establishes site-specific first-line treatment as a cornerstone in improving patient prognosis for cancer of unknown primary.
In 2021, Professor Zhiguo Luo designed conducted the prospective, single-arm phase II study (Fudan CUP-002) (ClinicalTrials.gov, NCT04848597) to evaluate the efficacy and safety of co-administration of F520 injection (anti-PD-1 antibody), bevacizumab, and nab-paclitaxel in patients with CUP who have progressed after first-line treatment. In this study, the objective response rate (ORR) was 54.2% (95% CI, 40.3 to 67.4), and the disease control rate (DCR) was 95.8% (95% CI, 86.0 to 98.9). These results demonstrate that second-line PD-1 blockade in combination with bevacizumab and nab-paclitaxel is an effective and well-tolerated treatment regimen for patients with CUP.
Moving forward, hot directions for future researches in cancer of unknown primary still focus on how to turn cancer of unknown primary into known primary by revolutionary diagnostic approaches, in order to guide therapeutic optimization.
Featured Image: In clinical practice, routine examinations for patients with cancer of unknown primary (CUP) include taking a medical history, conducting a physical examination, and detecting serum tumor markers. In addition, IHC or MTP-based methodologies were suggested to assist in identifying the tissue origin of CUP and guiding site-specific therapy for CUP. Image: Zhiguo Luo