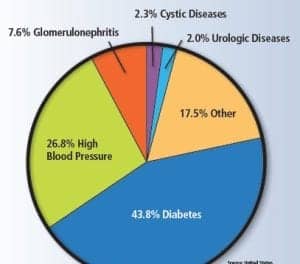
Abbott Laboratories, Abbott Park, Ill, has received 510(k) clearance from FDA for the Architect clinical chemistry hemoglobin A1c test, a tool to aid physicians in diagnosing and monitoring diabetes, as well as identifying people at risk for the disease. The HbA1c assay is used for the quantitative in vitro measurement of the percent hemoglobin A1c (% HbA1c) or the hemoglobin A1c concentration (mmol/mol) in human whole blood and hemolysate on Abbott’s Architect c8000 system. Hemoglobin A1c measurements are used to identify patients who may be at risk for developing diabetes mellitus, and for the monitoring of long-term blood glucose control in individuals with diabetes mellitus. Hemoglobin A1c should not be used for the diagnosis of diabetes in patients with abnormal red cell turnover, such as in cases of pregnancy, recent blood loss or transfusion, or some types of anemia. For more information, visit Abbott Laboratories.
HbA1c Test Receives FDA Clearance