Ellume has recalled its COVID-19 Home Test. The recall described in this notice is for the same issue that was announced in Potential for False Positive Results with Certain Lots of Ellume COVID-19 Home Tests Due to a Manufacturing Issue FDA Safety Communication on October 5, 2021.
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these tests may cause serious adverse health consequences or death.
Recalled Product
- Product Names: Ellume COVID-19 Home Test
- Product Codes and Lot Numbers: See Medical Device Recall Database Entries or the Ellume Recall Website below in the Additional Resources section
- Manufacturing Dates: February 24, 2021 to August 11, 2021
- Distribution Dates: April 13, 2021 to August 26, 2021
- Devices Recalled in the United States: 2,212,335
- Date Initiated by Firm: October 1, 2021
Device Use
The Ellume COVID-19 Home Test is an antigen test that detects proteins from the SARS-CoV-2 virus from a nasal sample in people two years of age or older. The Ellume COVID-19 Home Test is available without a prescription for use by people with or without COVID-19 symptoms. This at-home test uses swab samples taken from further up inside the nose (mid-turbinate) but not as deep inside the nose to reach the back of the throat (nasopharyngeal) where a health care professional collects a sample. The Ellume COVID-19 Home Test uses an analyzer that connects with a smartphone app to show users how to perform the test and understand the test results.
The FDA issued an Emergency Use Authorization (EUA) on December 15, 2020 and authorized a revision to the EUA on February 11, 2021 to allow emergency use of the Ellume COVID-19 Home Test.

Reason for Recall
Ellume is recalling certain lots of the COVID-19 Home Test because they have higher-than-acceptable false-positive test results for SARS-CoV-2. The reliability of negative test results is not affected. For these tests, a false positive test result shows that a person has the virus when they do not have it and could lead to:
- Delayed diagnosis or treatment for the actual cause of the person’s illness, which could be another life-threatening disease that is not COVID-19.
- Further spread of the SARS-CoV-2 virus when presumed positive people are grouped into cohorts (that is, they are housed together) based on false test results.
- The person receiving unnecessary COVID-19 treatment from a health care provider, such as antiviral treatment, convalescent plasma, or monoclonal antibody treatment, which can result in side effects.
- Disregard for the recommended precautions against COVID-19, including vaccination.
- Isolation, including monitoring household or close contacts for symptoms, limiting contact with family or friends, and missing school or work.
There have been 35 reports of false positive results sent to the FDA and no deaths reported.
Who May be Affected
People who received a positive test result for SARS-CoV-2 detected by the Ellume COVID-19 Home Test.
What to Do
On October 1, 2021, Ellume sent all impacted customers an “Urgent: Medical Device Recall” letter requesting they:
- Remove affected products from shelves and stop sales.
- Quarantine the affected products immediately.
- Contact their Ellume sales representatives for more instructions on disposal.
- Complete and send the acknowledgement form that was included with the recall letter to [email protected].
- Share the recall letter with any accounts or additional locations, if the affected products have been further distributed.
- Immediately notify Ellume of any accounts or additional locations that may have received the affected products.
Ellume is advising test users and caregivers to:
- Check if their product is from an affected lot. If their product is affected, visit www.ellumecovidtest.com/returnExternal Link Disclaimer for more instructions or call 1-888-807-1501 or email [email protected].
- Contact their health care provider, urgent care facility, or other COVID-19 testing site and request a COVID-19 molecular diagnostic test to confirm the positive test result if they used a test from one of the affected lots in the last two weeks and have not already had a follow-up molecular diagnostic test to confirm the positive test result.
The FDA issued Potential for False Positive Results with Certain Lots of Ellume COVID-19 Home Tests Due to a Manufacturing Issue FDA Safety Communication on October 5, 2021 recommending that test users and caregivers:
- Contact your health care provider, urgent care facility, or other COVID-19 testing site if:
- You received a positive test result using one of the affected lots of the Ellume COVID-19 Home Test more than two weeks ago, and
- You did not receive a positive result from a different COVID-19 test at the time of the original Ellume positive test result. A health care provider can help you decide what next steps you should take. You should not assume that you had COVID-19 or have immunity to COVID-19. You should continue to take recommended precautions, including vaccination, to avoid COVID-19 infection, following the Centers for Disease Control and Prevention’s guidelines.
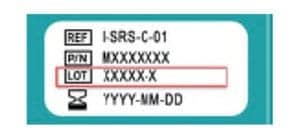
How to Recognize Affected Tests
Customers can find product lot numbers on the sticker on the side of the Ellume COVID-19 Home Test carton. Compare the lot number to the recalled lot numbers listed in the Medical Device Recall Database Entry. Affected tests purchased by consumers but not yet used will be disabled via a software update. Ellume will also inform customers who used an affected test and received a positive result.
Contact Information
Customers with questions about this recall should call Ellume at 1-888-807-1501 or email [email protected].
Additional Resources:
- Medical Device Recall Database Entry
- Safety Communication: Potential for False Positive Results with Certain Lots of Ellume COVID-19 Home Tests Due to a Manufacturing Issue
- Ellume Emergency Use Authorization
- Ellume Recall WebsiteExternal Link Disclaimer
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX.





