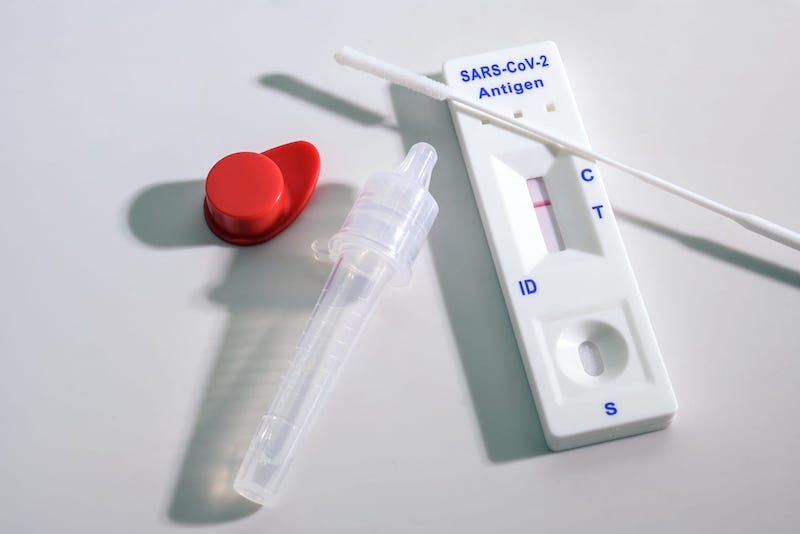
On Thursday, Nov. 17, 2022, the U.S. Food. and Drug Administration (FDA) issued emergency use authorizations for two COVID-19 Antigen Home Tests.
Th Hotgen COVID-19 Antigen Home Test is an over-the-counter (OTC) COVID-19 antigen home test that shows results in 15 minutes. The test can be used as a serial test for people within the first 7 days of experiencing COVID-19 symptoms or for individuals without symptoms. The antigen test should be repeated if a negative result is found – at least twice over 3 days with at least 48 hours between tests if the individual has symptoms, or at least 3 times over 5 days with at least 48 hours between tests if the individual does not have symptoms. The test can be used for people age 14 years or older with a self-collected nasal swab sample and age 2 years or older when an adult collects the nasal swab sample, according to the FDA.
The NIDS COVID-19 Antigen Home Test had its validation data gathered through the FDA’s collaboration with the National Institutes of Health Independent Test Assessment Program. The NIDS COVID-19 Antigen Home Test is an OTC COVID-19 antigen home test that shows results in 15 minutes. The test can be used as a serial test for people within the first 5 days of experiencing COVID-19 symptoms or for individuals without symptoms. The antigen test should be repeated if a negative result is found – at least twice over 3 days with at least 48 hours between tests if the individual has symptoms, or at least 3 times over 5 days with at least 48 hours between tests if the individual does not have symptoms. The test can be used for people age 14 years or older with a self-collected nasal swab sample and age 2 years or older when an adult collects the nasal swab sample.
As of November 2022, 437 tests and sample collection devices have been authorized by the FDA under emergency use authorizations (EUAs). These include 300 molecular tests and sample collection devices, 85 antibody and other immune response tests, 51 antigen tests and one diagnostic breath test. There are 78 molecular authorizations and one antibody authorization that can be used with home-collected samples. There is one EUA for a molecular prescription at-home test, two EUAs for antigen prescription at-home tests, 19 EUAs for antigen over-the-counter (OTC) at-home tests and four molecular OTC at-home tests.
The FDA has authorized 35 antigen tests and eight molecular tests for serial screening programs. The FDA has also authorized 1,161 revisions to EUA authorizations.