Ortho Clinical Diagnostics, Raritan, NJ, has been recognized with a prestigious silver 2021 Edison Award for its Vitros covid-19 testing solutions, helping labs meet demands of the pandemic with reliable mass-scale testing options.
The Edison Awards is an annual competition honoring excellence in new product and service development, marketing, design, and innovation. The awards committee recognized Ortho’s Vitros Anti-SARS-CoV-2 Total and IgG Antibody Tests and Vitros SARS-CoV-2 Antigen Test in the innovation subcategory for accelerated testing solutions after reviewing more than 7,000 products and services and over 400 nominations for new products and services development. This is Ortho’s fourth Edison Award.
“When the world needed highly accurate covid-19 diagnostic solutions, Ortho leveraged over 80 years of experience in infectious disease and quickly launched three high-quality covid-19 tests, enabling fast, accurate results healthcare teams, researchers, and government officials could trust,” says Chockalingam Palaniappan, PhD, chief innovation officer, Ortho Clinical Diagnostics. “We are honored to be recognized for our contribution to the pandemic and will continue to deliver solutions to help our customers and their patients understand this virus, immunity, and help aid reopening measures in the wake of vaccines.”
Ortho was the first company to launch high-volume covid-19 antibody and antigen tests with FDA emergency use authorization (EUA). In just 19 days, Ortho went from concept to launch of its covid-19 total antibody test. Two weeks later, Ortho received EUA for its covid-19 IgG antibody test. Both tests help clinicians understand whether a patient has been exposed to and developed antibodies to SARS-CoV-2 and offer 100% specificity, providing an extremely high level of confidence for both patients and clinicians, and have CE Mark.
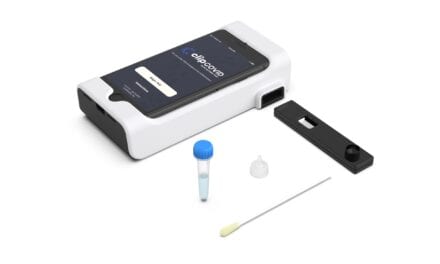
Ortho’s third covid-19 solution, the Vitros SARS-CoV-2 Antigen Test, helps confirm whether a patient has an active infection by detecting viral proteins in swab samples. The test, the first high-throughput antigen test with EUA, has utility for mass-scale testing and delivers same-day results to help hospitals and reference labs address issues of testing backlogs, supply shortages, and delayed results. The test also received CE Mark in November 2020.
“We were very impressed by the level of collaboration and discovery in this year’s entries,” says Edison Universe Executive Director Frank Bonafilia. “Somehow, while facing the unprecedented challenges of this global pandemic, companies around the world figured out how to work safely and smartly and still innovate at an award-winning level.”
For more information about the company, visit Ortho Clinical Diagnostics.
Featured image: Ortho Clinical Diagnostics has been recognized with a prestigious silver 2021 Edison Award for its Vitros covid-19 testing solutions, including the Vitros SARS-CoV-2 Antigen Test, pictured. (Courtesy, Ortho Clinical Diagnostics)