M. genitalium testing is difficult due to the low bacterial load typically available in patient samples. The approval of a NAAT for the diagnosis of this sexually transmitted infection will help protect the fertility of both female and male patients alike.
By Irene Stafford, MD, MS, and Erik Munson, PhD
Effective treatment of sexually transmitted infections (STIs) begins with an accurate diagnosis, and fortunatly evolving screening technology keeps expanding our abilities to test with greater sensitivity and accuracy. As these new screening improvements become available, it is imperative that they are incorporated quickly into diagnostic paradigms, so that patients are protected from the potentially severe consequences of untreated STIs, such as pelvic inflammatory disease (PID) and infertility.1 A nucleic acid amplification test (NAAT) for mycoplasma genitalium (M. genitalium) testing that was approved by the U.S. Food and Drug Administration in January 2019 is one such advancement in the diagnostic toolkit to protect patient health.2
Until the NAAT for M. genitalium was developed, it was difficult to detect the STI, even though it has been a known pathogen since the 1980s.3 This bacterium of class Mollicutes, which colonizes both male and female reproductive tracts,4 proved elusive due to its small size (less than 1 micron in length), minimal genome (580 kb), and very slow growth rate in culture. M. genitalium also lacks a cell wall, which renders certain antibiotic treatments ineffective—another reason why a more effective testing method was needed.4-6
Prevalence of M. Genitalium
Due to ineffective detection tools (prior to NAATs) for M. genitalium testing, the infection rate is likely under-reported.4 However, available data does lend some insight into its prevalence in patients in comparison to other, better studied STIs. It is currently estimated that M. genitalium prevalence is between 10% and 17% in both women and men, demonstrating a prevalence similar to chlamydia trachomatis (C. trachomatis) infection in many healthcare settings,7-9 with the greatest prevalence in patients 18 to 30 years of age (women, 11.7–26.2%; men, 14.1–15.1%).8 Pregnant women remain at risk for M. genitalium infection. In a study of 719 remnant genital samples collected from pregnant women, 5.7% were positive for M. genitalium, which is higher than the reported prevalence rate for neisseria gonorrhoeae infection (<1%).10 In a regional outpatient OB/GYN patient investigation, a >10% M. genitalium detection rate was observed.11 Moreover, >80% of M. genitalium detections did not involve co-infection with another STI agent. Similar findings were noted in a limited inpatient OB/GYN dataset. These data suggest that the omission of M. genitalium testing from STI screening panels may miss STIs in the OB/GYN population.11 Ultimately, with the availability of sensitive NAATs for M. genitalium, we can attain a more accurate view of this bacterium’s prevalence and better quantify its threat to the sexual health of the overall population.
Prioritizing Testing
Like many other STIs, M. genitalium left untreated can lead to adverse health consequences in both genders. In women, this bacterium is detected in those with clinical cervicitis (up to 30%), urethritis, and pelvic inflammatory disease (PID; up to 22%).12,13 PID is a serious disorder that can lead to permanent damage of reproductive organs and infertility.14 In pregnant women, M. genitalium has been associated with preterm birth and spontaneous abortion.15 In men, M. genitalium is detected in approximately 40% of those with persistent/recurring non-gonococcal urethritis (NGU)12,13 and to a lesser extent, prostatitis.13 NGU can lead to worsening infection and infertility.16 M. genitalium has also recently been linked, in both genders, to acquisition and transmission of other STIs, including HIV.17-20 Due to the prevalence of M. genitalium, the potential consequence of non-treatment, and the new availability of FDA-cleared NAATs for diagnosing infection with the organism, the Centers for Disease Control and Prevention (CDC) updated its STI guidelines regarding its diagnosis and treatment.
NAATs for M. Genitalium
The 2021 CDC STI Treatment Guidelines recommend that women with recurring cervicitis be tested for M. genitalium and that testing should also be considered for women with PID. Men who have persistent or recurring urethritis should also receive M. genitalium testing.13 As for how to test for M. genitalium, the CDC recommends that a NAAT is the most effective way to identify this infection.13 One of the challenges of M. genitalium testing is the low bacterial load associated with infection compared with other STIs.21 In women, the median M. genitalium infection load can be 100 times lower (5.7 x 104/swab) than the median C. trachomatis organism load (5.6 x 106/swab),22 and in men with symptomatic non-gonococcal urethritis (NGU), bacterial loads of M. genitalium (median 1.9 x 104 geq/ml) can be 30 times lower than for C. trachomatis (6.6 x 105 geq/ml).21 NAATs can also help to differentiate M. genitalium as it presents similarly to chlamydia and gonorrhea,7 and it is important to differentiate from these STIs because treatment of the underlying condition in cervicitis/PID is distinct.13
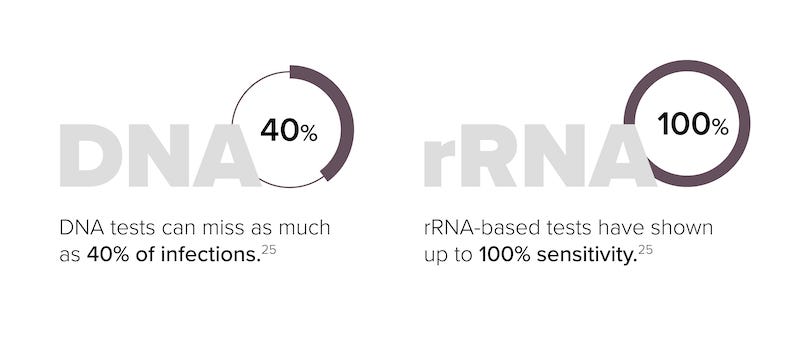
NAATs that target DNA or RNA sequences of M. genitalium have benefits over other testing methods for the organism. NAATs are significantly more sensitive than cell culture tests. It is also significantly faster—approximately 3 hours for a NAAT result versus up to 6 months for culture testing.23 Serologic tests have also proven to be suboptimal as well as inconclusive.13 The amplification of rRNA targets provides a potential advantage over DNA-based NAATs due to the fact that bacterial rRNA is present at up to 1,000 times the copy number of genomic DNA.24 The impact of genetic material abundance is highlighted by the accuracy of DNA- vs RNA-based tests, where DNA-based tests can miss up to 40% of M. genitalium infections while RNA-based tests have shown up to 100% sensitivity.25
Accurate diagnoses are key to prescribing effective treatments—some antibiotics are better suited for treating specific STIs. For instance, some antibiotics (such as penicillin) work by targeting the cell walls of bacteria. But, as mentioned earlier, M. genitalium lacks a cell wall, making these treatments ineffective. There is also the danger of developing antibiotic resistance; the incidence of macrolide (azithromycin) resistance in M. genitalium has been rapidly rising. Better informed diagnoses can also impact treatment strategies—the CDC guidelines recommend that if M. genitalium testing is available, it should be performed, and the results should be used to guide therapy. For instance, pharmacokinetic data indicate that pursuing a multiday strategy of azithromycin administration instead of a single-dose strategy might prevent inducing resistance in M. genitalium infections.13 Using available NAATs for M. genitalium testing, healthcare providers can reduce the percentage of cases where this infection is misdiagnosed or non-diagnosed, and increase the utilization of recommended antibiotic treatments to enhance patient care and minimize adverse outcomes.
Beyond NAATs: Additional M. Genitalium Testing Needs
NAATs are an important improvement in M. genitalium testing, but there is still a need for additional diagnostic capabilities, such as in antimicrobial resistance (AMR) testing. Currently there are no FDA-cleared tests on the market; molecular tests for macrolide (i.e., azithromycin) or quinolone (i.e., moxifloxacin) resistance markers are not yet commercially available in the United States, and the latest CDC guidelines reference the need for them. However, molecular assays that incorporate detection of mutations associated with macrolide resistance are at least under evaluation and will hopefully gain approval and be available soon.26
M. genitalium affects many patients, but available data are likely underestimates due to the difficulty in culturing this bacterium and the previous lack of available tests with adequate sensitivity to accurately detect it. This infection, if left untreated, can lead to serious adverse consequences for both male and female patients, including potential infertility, which is why it is so important for healthcare professionals to know if their patients have M. genitalium infection.
The approval of a NAAT for M. genitalium testing has finally allowed for accurate and timely testing, guiding more effective treatment decisions. Testing laboratories are important partners in providing optimal healthcare, and performing NAATs for M. genitalium will not only help protect patient reproductive and sexual health, but it will also increase our knowledge about this bacterium that will help shape new strategies for fighting it.
About the Authors
Irene Stafford, MD, MS, is associate professor in the Dept of Obstetrics, Gynecology, and Reproductive Sciences McGovern Medical School at the University of Texas Health Science Center at Houston, Texas.
Erik Munson, PhD, is associate professor, Medical Laboratory Science at Marquette University, Milwaukee, Wis.
References
1. Mayo Clinic. Sexually transmitted diseases (STDs). September 21, 2021. https://www.mayoclinic.org/diseases-conditions/sexually-transmitted-diseases-stds/symptoms-causes/syc-20351240. Accessed March 18, 2022.
2. United States Food and Drug Administration. Press release. FDA permits marketing of first test to aid in the diagnosis of a sexually-transmitted infection known as Mycoplasma genitalium. January 23, 2019. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-first-test-aid-diagnosis-sexually-transmitted-infection-known-mycoplasma#:~:text=The%20Aptima%20Mycoplasma%20genitalium%20Assay,a%20doctor’s%20office%20or%20clinic. Accessed March 2, 2022.
3. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
4. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: should we treat and how? Clin Infect Dis. 2011;53(suppl 3):129-142.
5. Fraser CM, Gocayne JD, Venter JC, et al. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397-403.
6. Glass JI, Assad-Garcia N, Alperovich N, et al. Essential genes of a minimal bacterium. Proc Natl Acad Sci U S A. 2006;103:425-430.
7. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36:598-606.
8. Gaydos CA, Manhart LE, Taylor SN, et al. Molecular testing for Mycoplasma genitalium in the United States: results from the AMES Prospective Multicenter Clinical Study. J Clin Microbiol. 2019;57:e01125-19.
9. Getman D, Jlang A, O’Donnell M, et al. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
10. Stafford IA, Hummel K, Dunn JJ, et al. Retrospective analysis of infection and antimicrobial resistance patterns of Mycoplasma genitalium among pregnant women in the southwestern USA. BMJ Open. 2021;11:e050475.
11. Munson E, Bykowski H, Munson KL, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-mediated amplification using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-438.
12. WebMD. What is Mycoplasma genitalium? https://www.webmd.com/sexual-conditions/mycoplasma-genitalium. Accessed March 18, 2022.
13. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR. 2021;70:1-187.
14. Mayo Clinic. Pelvic inflammatory disease (PID). https://www.mayoclinic.org/diseases-conditions/pelvic-inflammatory-disease/symptoms-causes/syc-20352594. Accessed March 18, 2022.
15. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. CID. 2015:61.
16. Cleveland Clinic. Nongonococcal urethritis in men. May 21, 2021. https://my.clevelandclinic.org/health/diseases/4426-nongonococcal-urethritis-in-men. Accessed March 18, 2022.
17. Barker EK, Malekinejad M, Merai R, et al. Risk of HIV acquisition among high-risk heterosexuals with nonviral sexually transmitted infections: A systematic review and meta-analysis. Sex Transm Dis. 2022 Jan 14. doi: 10.1097/OLQ.0000000000001601. Epub ahead of print. PMID: 35034049.
18. Van Dijck C, De Baetselier I, Cuylaerts V, et al. Gonococcal bacterial load in PrEP users with Mycoplasma genitalium coinfection. Int J STD AIDS. 2022;33:129-135.
19. Brin C, Palich R, Godefroy N, et al. Clinical, epidemiological and therapeutic characteristics of Mycoplasma genitalium infection in a French STI center. Infect Dis Now. 2022;52:13-17.
20. Tu W, Li YY, Kuang YQ, et al. High prevalence of sexually transmitted infections and risk factors among HIV-positive individuals in Yunnan, China. Eur J Med Res. 2022;27:9.
21. Frolund M, Lidbrink P, Wikstrom A, et al. Urethritis-associated pathogens in urine from men with nongonococcal urethritis: a case-control study. Acta Derm Venereol. 2016;96:689-694.
22. Walker J, Fairley CK, Bradshaw CS, et al. The difference in determinants of Chlamydia trachomatis and Mycoplasma genitalium in a sample of young Australian women. BMC Infect Dis. 2011;11:35.
23. Manhart LE and Trent M. Mycoplasma genitalium: a review of current issues and challenges. Contemporary OB/GYN. July 2017.
24. Kirkconnell B, Weinbaum B, Santos K, et al. Design and validation of transcription-mediated-amplification nucleic acid amplification tests for Mycoplasma genitalium. J Clin Microbiol. 2019;57:e00264-19.
25. Le Roy C, Pereyre S, Henin N, et al. French prospective clinical evaluation of the Aptima Mycoplasma genitalium CE-IVD assay and macrolide resistance detection using three distinct assays. J Clin Microbiol. 2017;55:3194-3200.26. Centers for Disease Control and Prevention. Sexually transmitted infections treatment guidelines, 2021: Mycoplasma genitalium. July 22, 2021. https://www.cdc.gov/std/treatment-guidelines/mycoplasmagenitalium.htm. Accessed March 18, 2022.