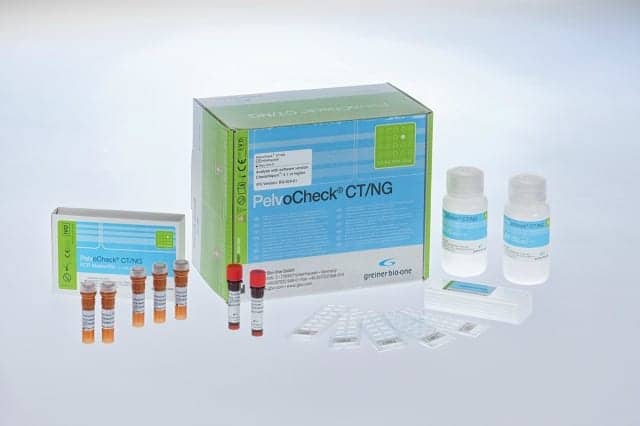
Developed for small and medium-sized laboratories, the PelvoCheck CT/NG diagnostic kit from Greiner Bio-One is especially useful for reliably testing asymptomatic patients for chlamydia and gonorrhoea infections.
The test reportedly delivers highly sensitive testing for Chlamydia trachomatis and Neisseria gonorrhoeae bacteria. Doctors advise sexually active women with multiple partners to test for these bacteria on an annual basis.
Two of the most common sexually transmitted diseases, chlamydia and gonorrhea do not immediately manifest to noticeable symptoms. As a result, an estimated 80% of chlamydia and 50% of gonorrhoea cases in women remain undiagnosed. If not treated, however, these infections often result in inflammation of the ovaries, fallopian tubes, or cervix, and lead to pelvic inflammatory disease. It can also give rise to complications during pregnancy or even infertility. Yet, when diagnosed early, the infections can be treated successfully with antibiotics.
With the PelvoCheck CT/NG kit, which is also validated for testing with pooled samples, up to 46 patient samples can be tested simultaneously for both Chlamydia trachomatis and Neisseria gonorrhoeae. Samples consist of cervical or vaginal smears or first-void urine. DNA is extracted with the help of the company’s oCheck DNA extraction kit and reproduced using polymerase chain reaction.
The amplification products are hybridized on a special microarray slide and analyzed with the CheckScanner to produce a customized, patient-specific report. According to the company, the diagnostic kit’s clinical sensitivity and specificity are very high. CE marked as an in vitro diagnostic in the European Union, and approved for screening programs in line with international regulations, PelvoCheck is part of the oCheck product range from Greiner Bio-One GmbH.
For more information, visit Greiner Bio-One.