How far has it come? Where does it need to go?
By Karen Appold
Despite advances in clinical laboratory testing devices, ongoing challenges continue to plague laboratorians when performing virology testing.
According to Lynnette Chakkaphak, MS, system laboratory director for St Vincent’s HealthCare, Jacksonville, Fla, and a board member of the American Society for Clinical Pathology (ASCP), the greatest current challenge in virology testing is achieving rapid turnaround times for samples from critically ill patients—even when the hospital’s own lab doesn’t perform the testing.
Chakkaphak offers the recent example of a critical situation that developed in the health system’s newborn nursery. Clinicians required same-day testing of all the infants in the nursery to determine whether they were witnessing an outbreak of respiratory syncytial virus (RSV) infection and, if so, which babies were infected. “The molecular instrument our laboratory used was not cleared by FDA for RSV testing,” Chakkaphak says. “But sending out the samples to a distant reference laboratory that batches tests on Mondays, Wednesdays, and Thursdays would have meant an unacceptably long turnaround time.”
In another instance, the health system had a critically ill patient who needed herpes simplex virus 1 and 2 polymerase chain reaction (PCR) testing on a sample of cerebrospinal fluid (CSF). In this case again, the physician expected same-day results for a test the hospital’s lab didn’t perform.
“Fortunately, in both cases, we were able to turn to another local medical center that has an extensive virology lab, and they were able to get these results to us in time,” Chakkaphak says. “Other labs might not have been so lucky and might have been forced to tell clinicians that rapid results were unavailable, possibly impacting patient outcomes.”
Paul E. Oefinger, PhD, senior director for immunology with Covance, Princeton, NJ, agrees that speed of testing is of utmost importance. “Comparability between laboratory-developed tests (LDTs) and point-of-care access is important because patients are increasingly receiving care in settings outside of hospitals,” he says. “While point-of-care is important for smaller clinical sites, rapid diagnostics allow high patient volumes to be served.”
Patrick Murray, PhD, worldwide director of scientific affairs for the diagnostic systems unit of BD Diagnostics, Sparks, Md, says viruses—particularly ribonucleic acid (RNA) viruses—can undergo rapid genetic changes that allow them to adapt to new hosts, including humans. This is best understood through the examples of influenza viruses and the coronaviruses that cause severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). Additionally, viruses such as West Nile virus and Dengue virus can be introduced into new geographic regions and to non-immune individuals. “These situations pose diagnostic and therapeutic challenges for both laboratorians and physicians,” he says.
In addition, a growing number of patients suffer from sepsis, serious pneumonia, or are immunocompromised and at high risk for infection. “Current diagnostic tools are often unable to identify the source of the infection in time to allow doctors to treat patients with appropriate, potentially life-saving therapies,” says Gavin Cloherty, PhD, associate director for scientific affairs at Abbott Molecular, Des Plaines, Ill.
Oefinger is also concerned about the mobility of diseases that can spread rapidly around the world; the rapid emergence of treatment-resistant virus strains; and new reassortment viruses, such as swine influenza, that may lead to rapidly spreading pandemics.
WHAT’S NEEDED MOST?
In addition to rapid turnaround time, what attributes are most sought-after for virology testing? Sensitivity? Specificity? Standardization and comparability? Point-of-care access?
Chakkaphak says that clinicians have come to expect high sensitivity and specificity for viral testing. “As manufacturers continue to offer advances in the automation of these complex procedures, the expectation that medical laboratory professionals will provide standardized results that can be compared globally has also grown,” she says.
While LDTs may once have been the gold standard in testing, commercial automated analyzers that simplify testing, save time, and provide greater reproducibility are rapidly growing in availability. FDA market clearance for many such analyzers has made it possible for smaller, less-sophisticated laboratories to provide viral testing.
While point-of-care access is not a priority for clinicians, speed of results is essential for patient care. Years ago, a clinician might have had few treatment options for a patient with a viral infection. With the new therapies that are now available, however, diagnostic testing that is both quick and accurate has become a vital part of the standard of care.
M. Robert Pawlak, PhD, genomics scientist for Covance, says that standardization in nomenclature is also desirable, especially regarding enteric viruses. Discrepancy exists between viral serotypes and genotypes, so that a single serotype may have several genotypes and vice versa. “With rapid mutational rates among viruses, molecular tests may become obsolete,” says Pawlak. “As new viral strains evolve, the new strains may not be detected.”
Cloherty believes that clinicians want assurance that molecular virology tests can pick up all viral strains with specificity, whatever their subtype or genotype, and that there is excellent sensitivity and precision at clinical decision points.
WHAT CUSTOMERS WANT
From a manufacturer’s perspective, Ryan Anderson, MBA, product marketing manager for BioFire Diagnostics, Salt Lake City, says customers want to rule-in or rule-out which etiologic agents are causing the presented symptoms faster and with more accuracy than traditional testing methods. Speed, comprehensiveness, sensitivity, and user-friendliness are all important factors when considering whether to adopt a new technology.
With the advancement of technology, co-infections are becoming more commonplace. “To help improve patient management, limit the spread of disease, and reduce overall healthcare costs, customers want systems that can detect multiple pathogens simultaneously,” says Anderson.
Antibiotic stewardship is a hot topic in hospitals, continues Anderson, especially with the recent release of the Centers for Disease Control and Prevention report, “Antibiotic Resistance Threats in the United States, 2013.” Hospitals continue to form antibiotic stewardship committees that seek ways to decrease the use of unnecessary antibiotics and treat patients more appropriately.
User-friendliness and benchtop space are also important for hospital laboratories, as they are being asked to do more with fewer resources. They want automated sample-to-result systems that are capable of running several applications, resulting in a one-stop shop, Anderson says.
FDA VERSUS LAB-DEVELOPED TESTS
According to Chakkaphak, LDTs have several distinct disadvantages compared to commercial FDA-approved tests. The first relates to the personnel that are required to perform such tests. The average hospital laboratory in the United States does not have a PhD clinical scientist or the highly specialized and experienced virologists that would be required to implement, validate, and properly monitor LDTs. The performance of such tests also requires much more time and effort. On the other hand, essentially all laboratories have on staff certified medical laboratory scientists (MLS ASCP) who are capable of instituting and managing viral tests that run on FDA-approved molecular diagnostics platforms.
Another disadvantage of LDTs is that their results are less reproducible and therefore less comparable than FDA-approved tests, especially when considering the automated instrumentation for viral testing that has received FDA approval. With FDA-approved tests, a lab is a member of a peer group that uses the same instrument and the same lot of reagent. Quality control data and analytical performance can be compared across the peer group to help ensure accurate and reproducible results.
Angela Caliendo, MD, PhD, professor and executive vice chair of medicine at the Warren Alpert Medical School of Brown University, Providence, RI, agrees, stating that FDA-cleared or approved tests are well defined, with similar results being obtained from different labs using the same test. “This is particularly important for viral load tests because they are standardized and the results are reliable,” she says. “Cost, however, is a problem, as well as the fact that they may be designed for batch testing when more rapid results are needed.”
Despite their disadvantages, sometimes LDTs are the only option. “There are many viral infections for which FDA-cleared or approved tests are not rapid or not available,” says Caliendo, including herpes simplex virus (HSV) encephalitis, varicella zoster virus (VZV) infections, Epstein-Barr virus (EBV), and BK virus. While HIV, hepatitis C virus (HCV), and hepatitis B virus (HBV) viral load tests are available, platforms that require batch testing and rather large batch sizes may not be practical for some labs. In these instances, a more moderate-sized instrument with random access would be helpful.
One advantage of LDT tests is that they can allow a laboratory to internally validate a test for multiple specimen types. Chakkaphak’s laboratory recently had a request for PCR testing on a bronchial washing specimen. Manufacturers of molecular equipment do not normally have FDA approval for this type of specimen, so LDTs would be a lab’s only option. These tests may also be less expensive.
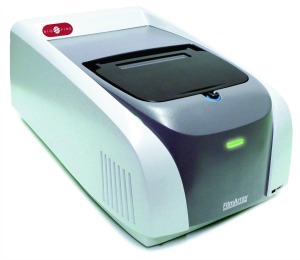
With only 2 minutes of hands-on time, the FDA-cleared FilmArray respiratory panel by
Biofire Diagnostics is capable of detecting up to 17 viral and 3 bacterial targets from a single patient sample in about an hour, with overall sensitivity of 95% and specificity 99%.
While automated platforms are moving viral testing further into the mainstream of laboratory tests, there is still some progress that needs to be made before a wide variety of viral tests become readily available to every laboratory. One drawback of current automated testing platforms is that they are limited in the number of viral agents for which they have FDA approval. For example, a laboratory may purchase an expensive analyzer that is capable of testing for human papillomavirus (HPV), but it will be unable to perform testing for influenza.
“If the laboratory has infectious disease specialists and pulmonologists who are increasingly requesting viral respiratory panels, then a third instrument might be needed,” Chakkaphak says. “In today’s laboratories, where capital dollars for these types of purchases are hard to come by, it is extremely difficult to meet the increasing demands of the clinician and their patient. Collaboration between our laboratory experts, pathologists, medical laboratory scientists, and clinicians should help us to win the healthcare dollars needed to move this ball in the right direction.”
Oefinger believes that a benefit of FDA-cleared commercial kits is that they continue to increase in quantity, covering a large array of human pathogenic viruses. LDTs are usually esoteric in nature and are only available at major academic institutions. “This is a disadvantage for patients who can only receive medical care at non-academic institutions,” he says. “Not all hospitals have access to all new diagnostic testing technology, due to expenses incurred or involved.”
In addition, reimbursement is less likely to be an issue with FDA-cleared kits, whereas it can be a serious sticking point for LDTs.
Murray says few laboratories are willing to develop and validate laboratory tests. “For this reason, the process for obtaining FDA clearance of commercially developed tests is critical, and it is welcome news that FDA has established streamlining the process of clearance as an important goal for 2014,” he says.
TESTING BEYOND THE HOSPITAL
Today’s medical laboratory is increasingly playing a vital role as part of a multidisciplinary medical team, advising clinicians on appropriate treatments for patients.
“To meet patients’ needs, we have to do the right tests at the right time for the right reason,” Chakkaphak maintains. “Our responsibility as lab professionals is to help clinicians select the most effective tests to get accurate diagnoses so clinicians can select the most efficient therapies.” As the numbers of patients being treated on an outpatient basis continues to rise, health systems are working to implement outreach laboratory programs or increase the size and reach of their current programs. This leads to a win-win situation in which the cost of patient care is kept low and the average cost per test decreases with increased testing volumes.
Many of the tests and platforms that are used in clinical virology diagnostics are not designed for use outside of the clinical laboratory, Caliendo notes. While technological advances such as rapid cartridge-based molecular platforms can be used outside of the laboratory, none are presently CLIA waived. More user-friendly devices are in development, but are not yet available.
In non-hospital healthcare facilities, the number of patients needing care for routine viral respiratory infections is expected to increase. “In this environment, the concern is that patients with severe viral respiratory infections may not receive proper therapeutic intervention in a timely fashion,” Pawlak says. “Hospital-based laboratories will be pressured to offer rapid diagnostic tests around the clock in order to assist with diagnosing severely ill patients who previously sought medical care outside of the hospital.”
For information about more virology testing solutions available for clinical laboratories, visit the online Tech Guides in the April issue of CLP.
Karen Appold is a contributing writer for CLP. For further information, contact chief editor Steve Halasey via [email protected].