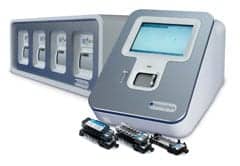
T2 Biosystems Inc. has submitted an application with the U.S. Food and Drug Administration (FDA) for Breakthrough Device Designation for the company’s Candida auris test. The company recently announced plans to add C. auris detection to its FDA-cleared T2Candida Panel.
Related: Timely Diagnosis: Improving Outcomes in Sepsis and Antimicrobial Resistance
Candida auris (C. auris) is a multidrug-resistant fungal pathogen with a mortality rate of up to 60% that has been labeled as a serious global health threat by the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO). The CDC has deemed C. auris as an urgent antimicrobial resistant threat, as it can be difficult to identify with standard laboratory methods, some strains are resistant to all three available classes of antifungals, it spreads easily in healthcare facilities, and can cause severe infections with high death rates. The CDC estimates the costs associated with U.S. fungal diseases, in general, are as high as $48 billion annually, and has called on public health professionals to help lower the burden of fungal disease by continuing to raise awareness of the life-saving benefits of early detection and proper treatment.
“We are pursuing FDA Breakthrough Device Designation for our novel direct-from-blood C. auris diagnostic test to potentially accelerate the path toward FDA clearance and commercialization,” says John Sperzel, chairman and CEO of T2 Biosystems. “Our T2Candida Panel is the only FDA-cleared diagnostic able to detect sepsis-causing fungal pathogens directly-from-blood, in just 3-5 hours, without the need to wait days for a positive blood culture. We believe adding C. auris to our T2Candida Panel will provide clinicians with rapid results, enabling targeted antimicrobial treatment, which is aligned with CDC’s call to action.”
The T2Candida Panel is the only FDA-cleared diagnostic test able to detect sepsis-causing fungal pathogens directly from whole blood, without the need to wait days for a positive blood culture, according to T2 Biosystems. The T2Candida Panel runs on the fully automated T2Dx Instrument and simultaneously detects five Candida species, including Candida albicans, Candida tropicalis, Candida parapsilosis, Candida krusei, and Candida glabrata. Rapid detection of these pathogens, as well as Candida auris, is essential to getting infected patients on appropriate antimicrobial therapy and improving clinical outcomes.
The FDA Breakthrough Devices Program, for which the C. auris diagnostic has been submitted, is a voluntary program for certain medical devices and device-led combination products that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. It is available for devices and device-led combination products which are subject to review under a premarket approval application (PMA), premarket notification (510(k)), or De Novo classification request (De Novo request). This program is intended to help patients have more timely access to these medical devices by expediting their development, assessment, and review, while preserving the statutory standards for PMA approval, 510(k) clearance, and De Novo marketing authorization, consistent with the FDA’s mission to protect and promote public health.