Verigene System
Benchtop workstation
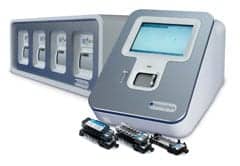
Nanosphere Inc, Northbrook, Ill, offers the Verigene® system, a benchtop molecular diagnostics workstation whose patented, gold-nanoparticle technology detects nucleic acid and protein targets. At its core are two lab instruments, the Verigene Reader and Processor, and Verigene test cartridges, which are single-use consumables. Users are guided through the testing process with intuitive software and a touch screen interface. The unit provides high sensitivity, accuracy, and rapid, multiplex target detection. Patient identification, sample identification, and test-cartridge tracking are done with a bar code, minimizing data entry and facilitating positive identification during testing. Sources are transferred once in the workflow, from a source tube into a test cartridge. Target nucleic acids are captured in a multiplex format, allowing for simultaneous assessment of a few to dozens of targets with high specificity. Random-access test processing and onboard controls allow users to test as needed without forced batching. Because it uses signal amplification instead of target amplification, contamination risk is minimal and samples may be prepared in the same workspace in which they are analyzed. No special training is needed, and the system operates in environments with limited bench space.
Nanosphere Inc
(888) 837-4436
www.nanosphere.us

Tissue of Origin Test
Aids in diagnosis of tumors of uncertain origin
Pathwork Diagnostics Inc, Sunnyvale, Calif, has introduced the Tissue of Origin Test through its CLIA-certified Pathwork® Diagnostics Laboratory. The new test aids in the diagnosis of tumors of uncertain origin and is among the first microarray-based diagnostic tests for cancer available in the United States. The test measures the expression of more than 1,500 genes to compare a tumor’s gene-expression profile to those of 15 known tissues, representing more than 60 morphologies, and to provide an objective, probability-based score for each potential tissue. It uses a proprietary Pathchip™ microarray and runs on the Affymetrix GeneChip® system. In a Pathwork clinical validation study of 487 metastatic and poorly differentiated and undifferentiated tumors that had already been identified using current methodologies, the test demonstrated a sensitivity of 89% and a specificity of 99%. Some 200,000 patients annually in the United States are found to have uncertain primary tumors, including cancer of unknown primary. Knowing the primary tumor site with greater certainty helps oncologists select cancer-specific therapy, according to National Comprehensive Cancer Network guidelines. Oncologists and pathologists may provide qualified frozen tissue specimens to the lab for processing and analysis with the test. A staff pathologist interprets the results and provides a comprehensive report to the ordering physician. The lab is licensed to accept samples from 45 states and is working to obtain licensing from the remaining five states (New York, Florida, Pennsylvania, Rhode Island, and Maryland).
Pathwork Diagnostics Inc
(408) 400-0828
www.pathworkdx.com

Prostate Px®+
Predicts prostate cancer progression/recurrence at diagnosis
Aureon Laboratories Inc, Yonkers, NY, has introduced Prostate Px® +, which predicts prostate cancer progression and disease recurrence at the time of diagnosis. The technology represents a new integrated approach known as systems pathology that combines molecular biomarkers, histological, and clinical information with advanced mathematics. At the time patients are diagnosed, the test forecasts disease progression after treatment, detects high-risk patients presenting as low-risk, reclassifies intermediate-risk patients, and helps identify those with less-aggressive disease. After a biopsy sample is sent to the company’s lab, the product analyzes the tissue, combining information from multiple sources to create a personalized, objective report about a patient’s prostate cancer. The test must be ordered by a physician. Urologists receive a report that includes a Px®+ SCORE™—a low of one to a high of 100—indicating the severity of disease and the likelihood of recurrence. According to the National Prostate Cancer Coalition, 218,890 new cases of prostate cancer were diagnosed in 2007 and an estimated 27,050 American men died from the disease. It is estimated that by 2015, more than 300,000 men will be diagnosed annually. The product is based on the results of a large study using data and samples from a cohort of 1,027 men assembled from the Mayo Clinic, Uppsala University, University of Connecticut, and Duke University Medical Center.
Aureon Laboratories
(888) 797-7284
www.aureon.com

xTAG Respiratory Viral Panel
Detect 12 viruses and viral subtypes
The xTAG™ respiratory viral panel from Luminex Corp, Austin, Tex, helps detect the presence or absence of several viruses and subtypes simultaneously in a few hours from a single patient sample. It is based on the company’s xMAP® multiplexing technology. The panel helps enhance virus surveillance and monitoring, reduce health care costs, and prevent inappropriate antibiotic use that has contributed to the creation of Superbugs. It has been cleared in the United States to detect 12 viruses and viral subtypes: adenovirus, influenza A (nonspecific subtype), influenza A H1, influenza A H3, influenza B, metapneumovirus, parainfluenza 1, parainfluenza 2, parainfluenza 3, respiratory syncytial virus (RSV) A, respiratory syncytial virus (RSV) B, and rhinovirus. In Europe, it is certified to detect and identify 20 viruses and viral subtypes, the viruses in the US panel plus: corona virus 229E, corona virus HKU1, corona virus NL63, corona virus OC43; enterovirus, influenza A H5, parainfluenza 4, and SARS. The test received 510(k) clearance from the FDA in January 2008 and its CE mark for sale in Europe in 2006.
Luminex Corp
(888) 219-8020
www.luminexcorp.com
Previstage GCC Colorectal Cancer Staging Test
Molecular assay
The Previstage™ GCC molecular assay from DiagnoCure Oncology Laboratories Inc, West Chester, Pa, identifies in paraffin-embedded, histopathologically negative lymph nodes, the expression of the guanylyl cyclase C (GCC) gene, and a biomarker that is normally found exclusively in the intestine. Research shows that when the expression of GCC mRNA is detected outside of the intestine—such as in the lymph nodes—it is a highly sensitive indication that colorectal cancer has spread. The histopathological status of the lymph node is the key prognostic criterion for recurrence of colon cancer, and despite best efforts, histopathology can miss metastases. Recurrence occurs in 25% to 30% of node-negative patients, indicating metastases were present but not identified by traditional methods. The test employs qRT-PCR technology, which has been shown to be up to 100,000 times more sensitive than traditional histopathology in detecting these occult metastases.
DiagnoCure Oncology Laboratories Inc
(888) 900-6626
www.diagnocurelabs.com