Summary:
datma’s federated real-world data marketplace, datma.FED, has surpassed one million records, offering pharmaceutical and research organizations secure access to high-quality genomic and pathology data without requiring centralized data transfers.
Key Takeaways:
- Scalable Access: datma.FED provides scalable, federated access to demographic, EHR, genomic, and pathology data, enhancing real-world research insights.
- Data Privacy & Security: The platform ensures HIPAA compliance and allows data custodians to retain control while enabling secure federated queries.
- Bridging Data Gaps: By integrating genomic and pathology insights, datma.FED helps pharma assess biomarker prevalence and refine market strategies.
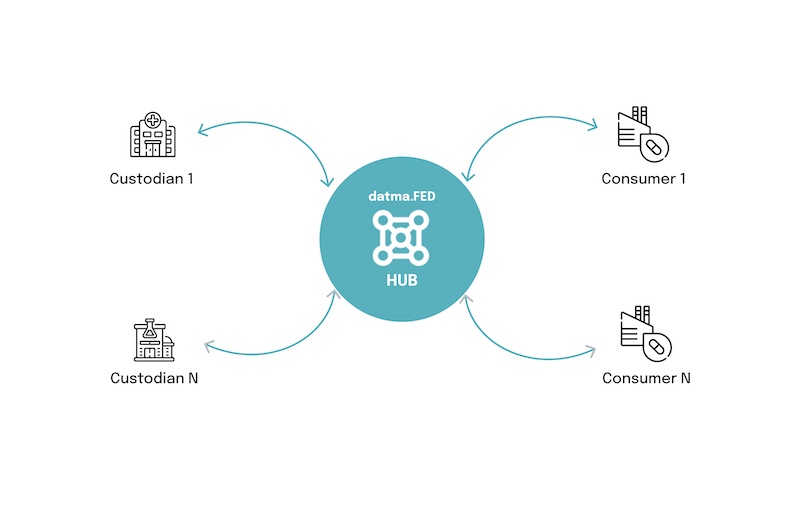
datma, a provider of federated real-world data access, has announced that its federated real-world data marketplace launched in October of 2024 has surpassed one million records available for research. This milestone demonstrates the strength of datma.FED as a platform for secure, federated access to high-quality, demographic, EHR, genomic, and pathology data, providing pharmaceutical and research organizations with critical insights often missing from traditional real-world data sources, according to the company.
datma.FED Provides Genomic and Pathology Insights
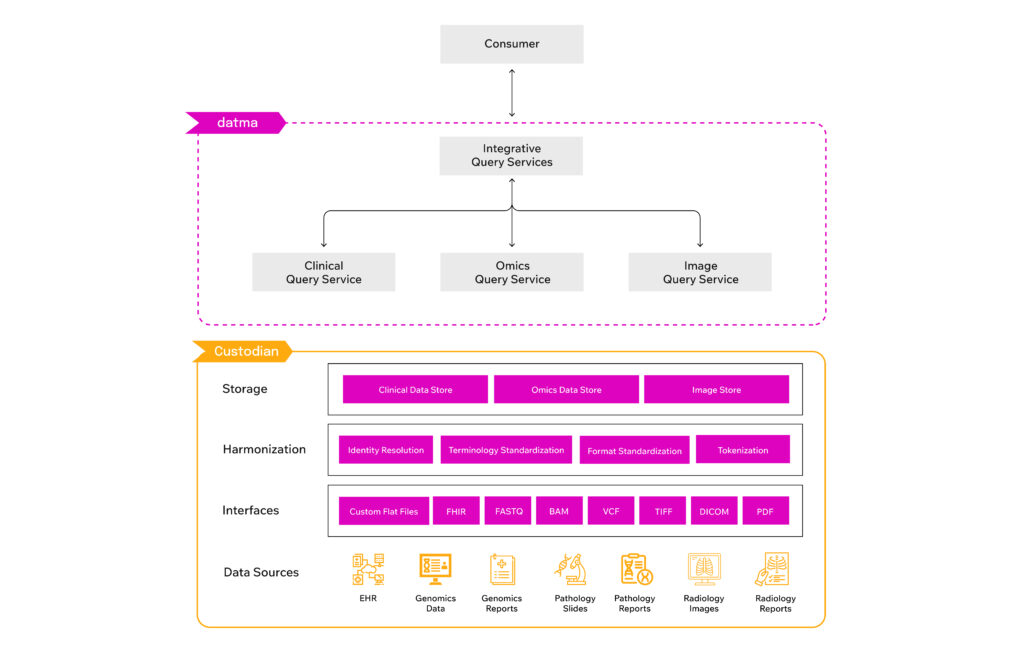
With the expansion of datma.FED, pharmaceutical and research organizations gain scalable access to contextual and longitudinal genomic and pathology insights. Unlike traditional data models that require data transfers or centralized repositories, datma.FED enables secure, federated queries, ensuring that data custodians retain full control over their datasets while providing pharmaceutical researchers access to real-world oncology data.
“This milestone significantly expands the depth and quality of real-world genomic data available within datma.FED,” says Noah Nasser, CEO at datma. “With over one million patient lives now available, researchers and pharmaceutical teams can gain deeper insights into real-world data and explore patterns in genomic testing utilization- while ensuring the highest privacy and data stewardship standards. By connecting the right data with the right researchers, we are accelerating discovery without the risks and challenges of traditional data-sharing models.”
Filling the Gap in Real-World Datasets
The integration of specialized molecular profiling and comprehensive genomic datasets fills a critical gap in pharma’s existing real-world datasets. While claims and EHR data provide insight into diagnoses and treatments, they often lack molecular testing details. By incorporating genomic and pathology data, pharma can now assess biomarker prevalence, evaluate molecular testing utilization trends, and identify gaps in testing uptake. These insights help pharma teams refine market access strategies and drive more informed commercialization efforts.
datma.FED’s federated model ensures compliance with HIPAA and regulatory standards while eliminating the burdens of manual data preparation and integration. The platform ingests, standardizes, and harmonizes healthcare data, making research-ready data immediately queryable for pharmaceutical and research teams. As datma continues to expand its network, it remains focused on bridging the gap between real-world data and its application in research and discovery in precision medicine.
Featured Image: datma.FED offers full control and protection of multi-omic and pathology data, according to the company. Image: datma