 |
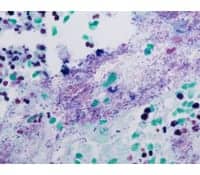
| Gram positive and gram negative bacteria with green counterstain, stained using VENTANA Gram Staining Kit on the BenchMark Special Stains automated staining platform. |
Ventana Medical Systems Inc, Tucson, Ariz, a member of the Roche Group, has released the VENTANA Gram Staining Kit1 worldwide.
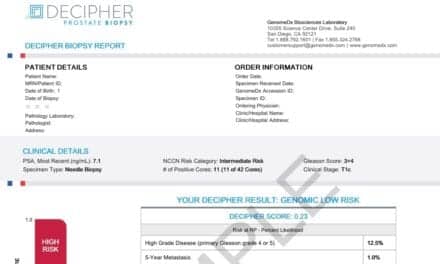
Automated for use on the VENTANA BenchMark Special Stains instrument (pictured below, left) the Gram Staining Kit aids pathologists in the most basic classification of bacteria into Gram-positive and Gram-negative bacteria in fixed tissue samples. Gram stain classification is clinically useful as it provides an initial indication of the nature of a patient’s infection.
 |
| Gram positive and gram negative bacteria with tartrazine counterstain, stained using VENTANA Gram Staining Kit on the BenchMark Special Stains automated staining platform. |
The Gram stain is part of a family of histochemical special stains that many labs around the world still run manually today. In automating the stain, the company gives its customers an efficient and automated method to perform this assay, enabling them to achieve increased productivity, consistency, and testing quality.
“Tissue diagnostics play a critical role in enabling accurate diagnosis and treatment decisions for cancer patients,” says Mara G. Aspinall, president, Ventana Medical Systems, adding that the company is empowering its customers to achieve greater efficiencies by automating common, yet labor-intensive tests such as the Gram stain.

The new kit provides visual consistency and clarity rarely achieved with manual Gram staining protocols, says Adrian Ralph, vice president, Primary Staining, Ventana Medical Systems.
Click here to learn more about the Special Stains instrument and watch a time-lapse video.
1. The VENTANA Gram Stain Kit is being launched as a US Class I exempt/ CE-IVD product.
[Source: Ventana Medical Systems]