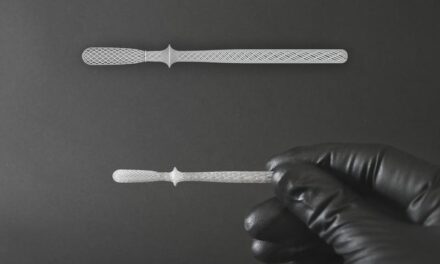
Sterile ultra-thin nasal and nasopharyngeal specimen collection swabs from Trinity Biotech, Bray, Ireland, are ergonomic and anatomic in design to improve patient comfort as well as provide optimum specimen collection. The FDA-registered swabs are compatible with rapids, EIA, molecular, DFA, cytology, bacteriology, and viral culture applications and are individually packaged in paper peel pouches for easy removal and use. They feature a safe and convenient breakpoint for handling and transportation as well as an acrylonitrile butadiene styrene (ABS) shaft and 100% nylon tip. The swabs are for use with the majority of media tubes and Trinity Biotech’s Bartels Flex Trans media collection kit. For more information, visit Trinity Biotech.
Ultra-Thin Nasal and Nasopharyngeal Flocked Swabs