With the increased clinical utility of sub-99th percentile hs-cTn measurements, quality controls with lower cardiac troponin concentrations are needed to monitor and validate hs-cTn assay performance.
By Curtis Daly, Alexandria Brooks, and Jessica McKenna
Cardiac troponins (cTn), cardiac troponin I (cTnI) and cardiac troponin T (cTnT), are important analytes for acute myocardial infarction diagnosis and are used to guide treatment and patient management decisions. Recent approvals of high sensitivity cardiac troponin (hs-cTn) assays by the U.S. Food and Drug Administration (FDA) and international regulatory bodies have enabled clinical labs to develop more sophisticated diagnostic protocols based on utilization of low cTn measurements at or near the assays’ limit of detection (LoD) to expediate and improve the accuracy of acute myocardial infarction diagnosis. The following is a concise review of assay sensitivity as it relates to hs-cTn testing, it’s relevance to clinical labs for subsequent diagnosis of cardiac events, and conclude with high level considerations for cTn target concentrations for quality controls intended to monitor the performance of hs-cTn assays.
Assay Analytical Sensitivity and Clinical Upper Reference Limit Terms
Table 1 provides a brief overview defining the terms used in this article.
| Table 1. Definition of LoB, LoD, LoQ, According to Clinical Laboratory Standards Institute (CLSI) Document EP17-A2 (1) and 99th Percentile URL. | |
| Term | Definition |
| Limit of Blank (LoB) | The LoB represents the highest analyte concentration expected to be reported when replicates of a blank sample containing no analyte are tested. The LoB is always less than the LoD and the LoQ. |
| Limit of Detection (LoD) | The LoD is defined as the lowest actual analyte concentration that can reliably be distinguished from the LoB. The LoD is determined by testing replicate samples containing low concentrations of the analyte. The LoD is always greater than the LoB. |
| Limit of Quantification (LoQ) | The LoQ is the lowest analyte concentration at which the analyte is reliability detected in accordance with predefined goals for imprecision or bias. The LoQ may be equal to or greater than the LoD. |
| 99th Percentile Upper Reference Limit (URL) | The 99th percentile represents the analyte concentration (for which only 1% of the population has higher levels in a healthy reference population. 99th percentiles for cardiac Troponin I and T are often used as cut-off values for diagnosing AMI. 99th percentiles for cardiac troponin assay methods addressed in this article are greater than their respective assay methods’ LoB, LoD, and LoQ. |
Increased Clinical Reliance on Low Level Cardiac Troponin Concentrations for Diagnosis and Treatment
Non-high sensitivity cardiac troponin assays lack the analytical sensitivity to reliably measure small cTn concentrations and their subsequent changes below the 99th percentile upper reference limit (URL). Assays for hs-cTnI and hs-cTnT have allowed clinical labs to shift emphasis from using cardiac troponin as binary tests (in which the 99th percentile URL of a healthy reference population was used as a cut-off for defining normal vs increased cTn values) to more complex diagnostic protocols utilizing cTn values below the 99th percentiles’ URLs. More specifically, values representative of the assays limit of detection (LoD) and limit of quantification (LoQ), have functioned as cutoffs for early rule-out of acute myocardial infarction testing protocols4.
The ability of hs-cTn assays to measure low cTn values with precision can support the early and safe discharge of patients based on a single cTn measurement combined with additional clinical assessment or through using serial testing protocols that monitor small changes in sub-99th percentile URL cTn concentrations over a shorter sampling time interval3, 4, 5. Since lower concentrations of cTn are being used to make clinical decisions, labs are expected to perform additional validation of the assays’ LoB, LoD, and LoQ parameters to instill confidence in the assay performance at these low concentrations5. Correspondingly, appropriately targeted quality control materials for validation and monitoring of assay performance are vital to minimize reporting of incorrect patient results that would impact patient care.
Table 2 below presents analytical characteristics of some FDA approved hs-cTnI assays on major IVD platforms from Abbott, Beckman Coulter, Siemens, and hs-cTnT assay from Roche according to assay manufacturer information compiled by the International Federation of Clinical Chemistry (IFCC)2 . Notably, sex-specific and overall 99th percentiles show considerable differences within and between assays, with males presenting higher 99th percentiles than females, reflecting the longstanding lack of standardization that can impact hsTnI assays and resulting patient outlook5. Assay performance requirements for hsTn as it relates to imprecision from the International Federation of Clinical Chemistry on Clinical Application of Cardiac Bio-Markers (IFCC TF-CB) has established an analytical requirement for hs-cTn assays to have a percent co-efficient of variation (%CV) at the 99th percentile of less than or equal to 10%, a standard for imprecision that is also included in the Fourth Universal Definition of Myocardial Infarction (UDMI), and recommend the LoD as the lowest reportable limit. In contrast, the FDA allows reporting of hs-cTn assays to the LoQ, typically the concentration where the CV is 20%5.
Analytical Characteristics of Representative hs-cTn Assays from Abbott, Beckman-Coulter, Siemens, and Roche
| Table 2. Analytical Characteristics of Select l hs-cTnI and hs-cTnT Assays | |||||||
| Company/ Platform/ Assay | Analyte | LoD (pg/mL) | Conc at 20% CV (pg/mL) | Conc at 10% CV (pg/mL) | % Normals Measured ≥ LOD: Female, Male, Overall | 99th Percentile: Female, Male, Overall (pg/mL) | % CV at 99th Percentile |
| Abbott/ ARCHITECT i systems/ ARCHITECT STAT High Sensitive Troponin-I | hs-cTnI | 1.7 | 2.3 | 4.6 | Female: 78% Male: 92% Overall: 85% | Female: 17 Male: 35 Overall: 28 | Female: 5.0% Male: 4.1% Overall: 4.3% |
| Beckman Coulter/ DxI, Access hsTnI | hs-cTnI | 1.5 to 2.3 | 1.2 to 2.3 | 5.6 | > 50% | Female: 13.6 Male: 19.8 Overall: 18.1 | Female: 6.5% Male: 6.1% Overall: 6.2% |
| Siemens ATELLICA High-Sensitivity TnI (TnIH) | hs-cTnI | 1.6 | 2.5 | <6.0 | Female: 62% Male: 89% Overall: 75% | Female: 38.6 Male: 53.5 Overall: 45.4 | <4.0% |
| Roche/ cobas e601, e602, E170/ TnT Gen 5 STAT | hs-cTnT | 3 | 6 | 11 | Overall: 55.1% | Female: 14 Male: 22 Overall: 19 | <10% |
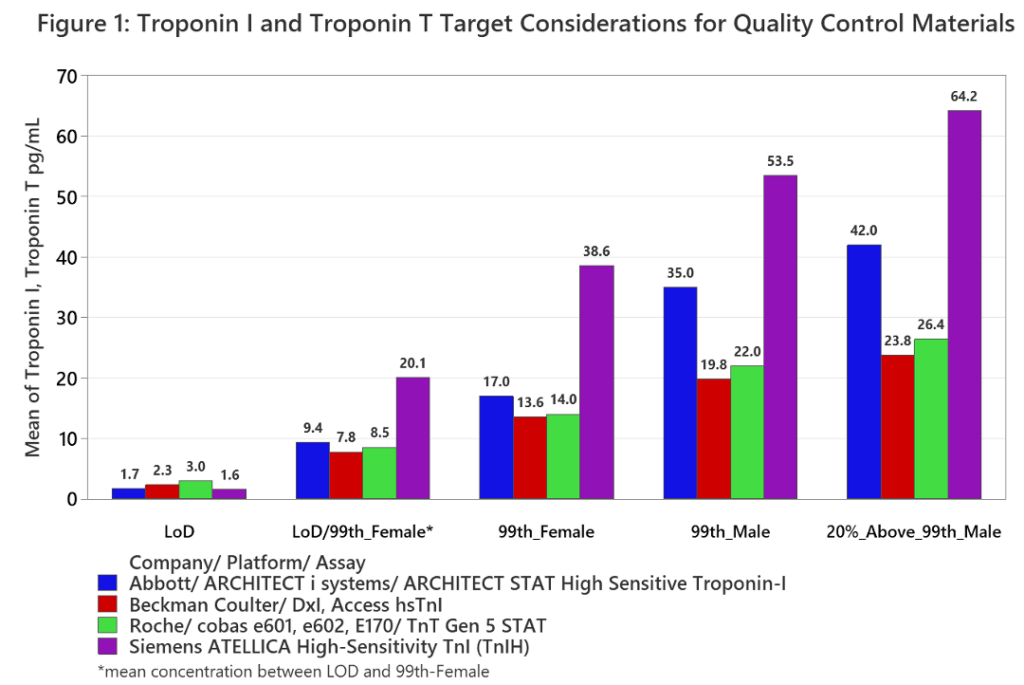
Table 3 presents recommendations from AACC and IFCC collaborations applicable to quality controls for hs-cTn assays with Figure 1 depicting quality control concentration recommendations B), C), and D) with respect to the hs-cTn assays’ applicable analytical
characteristics. In cases where the LoD is expressed as a range, only the upper value was graphed.
| Table 3. Recommendations for Quality Control hs-TnI and hs-cTnT Target Concentrations |
| A) Laboratories should measure at least 3 different concentrations of QC materials5 |
| B) Concentrations should be consistent with the LoD of each hs-cTnI and hs-cTnT assay2 |
| C) Concentrations should be between the LoD and the lowest sex-specific 99th percentile5 |
| D) Concentrations should be consistent with both the male and female sex-specific upper reference limits2 |
| E) Concentrations should include one that challenges the assays’ upper analytical range of reportable cTn results5 |
hsTnI and hsTnT Quality Control Materials: Opportunities and Challenges
With the increased clinical utility of sub-99th percentile hs-cTn measurements, quality controls with lower cardiac troponin concentrations are needed to monitor and validate hs-cTn assay performance. However, it is important to note that manufacturing quality controls close to the assays’ LoD and LoQ is impacted by a number of challenges and considerations:
- LoD and LoQ concentrations are expected to have %CV’s ≥ 20% which impairs consistent and accurate targeting during manufacturing which can be seen in Table 2 above.
- Quality Control Results < LoD are highly likely to arise due to reasons including, but not limited to:
- Proteolytic and other analyte degradation mechanisms
- Variation in quality control pre-analytical handling and storage conditions
- Measurement variance from reagent lot changes and calibration shifts
- Lack of hs-cTnI assay standardization impairs the ability to make universal quality controls with the same clinical utility across multiple methods. To minimize the extent of quality control levels with assay manufacturer specific cTnI concentrations, cTnI raw material antigens capable of being measured across multiple assays in the same relative proportion as the assays’ reported 99th percentile URLs will need to be identified and sourced.
Due to the challenges described above, targets between the LoD and the lowest sex-specific 99th percentile upper reference limit may be of more value in lieu of at or near the assay LoD. Thus, a minimum of four quality control concentration levels may warrant being manufactured to support QC laboratory needs. One between LoD and female 99th, the female 99th, the male 99th, and a high concentration to challenge the assays upper reportable limit with the possibility of more levels needed depending on the extent of challenge encountered by the lack of assay standardization.
About the Authors

Curtis Daly, MS, MBA, is a senior staff scientist at Bio-Rad Laboratories.

Alexandria Brooks, is a senior scientist at Bio-Rad.

Jessica McKenna, is an R&D Research Associate at Bio-Rad Laboratories
References
- Clinical and Laboratory Standards Institute (CLSI). Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures; Approved Guideline—Second Edition. CLSI document EP17-A2 (ISBN 1-56238-795-2 [Print]; ISBN 1-56238-796-0 [Electronic]). Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite2500, Wayne, Pennsylvania 19087 USA, 2012.
- High-Sensitivity* Cardiac Troponin I and T Assay Analytical Characteristics Designated by Manufacturer IFCC Committee on Clinical Applications of Cardiac Bio-Markers (C-CB) v092021 (no date). Available at: https://ifcc.web.insd.dk/media/479205/high-sensitivity-cardiac-troponin-i-and-t-assay-analytical-characteristics-designated-by-manufacturer-v092021-3.pdf (Accessed: May 3, 2023).
- Atul Anand, Anoop S V Shah, Agim Beshiri, Allan S Jaffe, Nicholas L Mills, Global Adoption of High-Sensitivity Cardiac Troponins and the Universal Definition of Myocardial Infarction, Clinical Chemistry, Volume 65, Issue 3, 1 March 2019, Pages 484–489, https://doi.org/10.1373/clinchem.2018.298059
- Fred S Apple, Corinne R Fantz, Paul O Collinson, the IFCC Committee on Clinical Application of Cardiac Bio-Markers, Implementation of High-Sensitivity and Point-of-Care Cardiac Troponin Assays into Practice: Some Different Thoughts, Clinical Chemistry, Volume 67, Issue 1, January 2021, Pages 70–78, https://doi.org/10.1093/clinchem/hvaa264
- Alan H B Wu, Robert H Christenson, Dina N Greene, Allan S Jaffe, Peter A Kavsak, Jordi Ordonez-Llanos, Fred S Apple, Clinical Laboratory Practice Recommendations for the Use of Cardiac Troponin in Acute Coronary Syndrome: Expert Opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine, Clinical Chemistry, Volume 64, Issue 4, 1 April 2018, Pages 645–655, https://doi.org/10.1373/clinchem.2017.277186