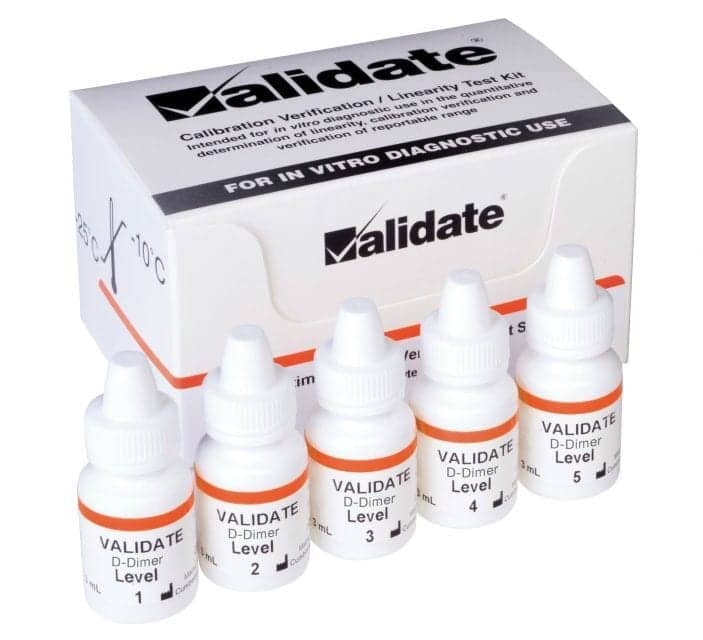
The Validate D-Dimer calibration verification and linearity test kit from LGC Maine Standards, Cumberland Foreside, Maine, has received FDA premarket notification (510(k)) clearance for use on Stago STA-R Evolution analyzers. The kit evaluates D-Dimer in a human plasma base. Each kit is prepared using the Clinical and Laboratory Standards Institute recommended “equal delta” method for linearity testing and is liquid, ready-to-use. Users simply add the product from the dropper bottle directly into a sample cup and run in replicates. The company’s MSDRx data reduction software is available at no charge for real-time data analysis. In the United States, a laboratory can send data to LGC Maine Standards, where a technical specialist will complete the data analysis and return a report within 5 business days. For more information, visit LGC Maine Standards.
D-Dimer Calibration Verification, Linearity Test Kit for Stago Analyzers