DiaSorin Inc
(800) 328-1482
www.diasorin.com
LIAISON® Treponema Assay
DiaSorin introduces the LIAISON® Treponema Assay, the first fully automated FDA cleared chemiluminescent immunoassay to aid in the diagnosis of syphilis infection.
With high sensitivity as well as excellent specificity, the LIAISON® Treponema Assay can be used as a clinical laboratory screening test or a confirmatory test. Its ability to detect both IgM and IgG antibodies to Treponema pallidum ensures improved sensitivity in the diagnosis of early syphilis infections compared to RPR and EIA methods. The use of recombinant antigens limits cross-reactivity and provides superior specificity. In addition, laboratories will benefit from the excellent throughput of 180 results per hour and the total walk-away system.
Euroimmun US
(800) 913-2022
www.euroimmunus.com
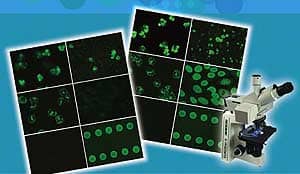
MultiPlex IFA ANCA Testing
The Euroimmun 6 BIOCHIPs ANCA test offers significant advantages in turnaround time (less than 2 hours) and cost. Laboratory can simultaneously screen and confirm the patterns associated with c- ANCA and p-ANCA, as well as atypical p-ANCA. Each well contains 6 individual substrates: (1) ethanol fixed granulocytes, (2) formalin fixed granulocytes, (3) HEp-20-10 cells, (4) primate liver, (5) PR3 antigen dots and (6) MPO antigen dots. The granulocytes substrates are utilized in the initial reading of c-ANCA and p-ANCA. The HEp-20-10 substrate is useful in confirming co-existing ANA, and its inclusion eliminates the necessity to run an additional assay. The PR3 and MPO dots are useful in confirming c-ANCA or p-ANCA patterns.


