The ERG oncoprotein from BioCare Medical LLC is a promising diagnostic marker for identifying prostatic adenocarcinoma and distinguishing it from non-neoplastic prostate and other adenocarcinomas.
ERG translocation is the molecular hallmark of a new class of prostate cancer.” This statement, made by a lead pathologist at a major California anatomic pathology reference laboratory, was our first introduction to ERG fusion. This new marker identifies a gene fusion (or translocation) that is present in 50% to 70% of all prostate carcinomas.1 The recent discovery of gene translocations in prostate cancers calls for a paradigm shift in our understanding of the molecular basis of this disease.
Transcriptional regulator ERG (ETS Related Gene) is a protein that in humans is encoded by the ERG gene. ERG is a member of the ETS family of transcription factors. Transcriptional regulator ERG is a nuclear protein that binds purine-rich sequences. Transmembrane protease, serine 2 (TMPRSS2) is an enzyme that in humans is encoded by the TMPRSS2 gene. ERG can fuse with TMPRSS2 protein to form an oncogenic fusion gene that is commonly found in human prostate cancer, especially in hormone refractory prostate cancer. This suggests that ERG overexpression may contribute to development of androgen independence in prostate cancer through disruption of androgen receptor signaling.
The TMPRSS2-ERG fusion leads to induction of the ERG [proto]oncogene, adding an important piece to the puzzle of prostate cancer diagnosis. According to Albert Dobi, PhD, Chief, Section for Gene Regulation and Bioinformatics, Center for Prostate Disease Research (CPDR) and the Henry M. Jackson Foundation for the Advancement of Military Medicine in Rockville, Md, “… the CPDR ERG-MAb opens the avenues for … ERG oncogenic pathway-targeted therapy in more than half of prostate cancers, which is similar to oncogene-targeted therapies—HER2, EGFR, BCR-ABL, PDGFR—currently being used in other cancers.”
ERG oncoprotein expression has been shown to be exquisitely specific for prostate cancer. Major studies have demonstrated a specificity of 99.9% for detection of prostate cancer.1 With this level of specificity, if you have an ERG positive sample, you very likely have cancer.
The clinical usefulness of ERG fusion detection by immunohistochemistry (IHC) extends beyond differentiation of benign from cancerous tissue. About 1.3 million prostate biopsies are performed in the United States per year. Since only a small portion of the prostate can be sampled, it has been reported that the sensitivity of prostate biopsy for detection of prostate cancer is limited. Patel et al showed that 38% of prostate cancer was missed by biopsy,2 while Haas et al demonstrated that 20% of clinically significant cancers were missed by prostate biopsy.3
IMPORTANT CLUES
ERG provides an important clue to pathologists and urologists with the potential to increase the sensitivity of prostate biopsy, even if the cancer is not found in biopsy samples. According to research by Dobi and Isabel Sesterhenn, MD, Chair of Genitourinary Pathology at the Armed Forces Institute of Pathology, detection of ERG by IHC in high-grade prostatic intraepithelial neoplasia (HGPIN) is associated with the index tumor in 90% of cases, even if prostate cancer is not found in the biopsy. HGPIN is not considered cancer. However, the presence of ERG in this non-cancerous tissue appears to indicate the presence of cancer elsewhere in the prostate. Since the index tumor is typically the largest tumor and the one that is driving the course of the disease, detection of ERG positive PIN may provide diagnostic information indicating the need for more aggressive patient follow-up and subsequent biopsy.
Other studies have shown a strong concordance of ERG positive PIN with ERG positive carcinoma in prostatectomies. FISH tests are also available that detect the ERG fusion. However, these can be expensive and time consuming. Immunohistochemistry is a simpler technique, and 90% correlation has been demonstrated between ERG fusion FISH and an immunohistochemistry method. This study used ERG monoclonal antibody clone 9FY, which was developed by the Center for Prostate Disease Research in association with the Henry M. Jackson Foundation, and is under exclusive license by BioCare Medical LLC, Concord, Calif.
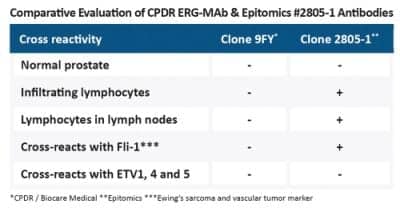
Few antibodies are available that detect the ERG translocation via immunohistochemistry in tissue specimens. When evaluating antibodies, the key question to address is: What is the specificity? Specificity differences such as cross reactivity with related ETS family transcription factors such as Fli-14 and ETV are critically important. Furthermore, the absence of cross reactivity to T- and B-cells is critical, as this makes evaluation of tumor of unknown origin in lymph nodes by presence of ERG much more difficult.
ETS-family transcription factors such as ERG, Fli-1, ETS-1, NERF-2, and TEL are also expressed in vascular endothelial cells, and are sensitive markers for acute myeloid leukemia (AML) and Ewing’s Sarcoma, so can provide assistance in these critical diagnoses.
MULTIPLEX IHC APPLICATIONS
By using Multiplex IHC technologies, ERG can also be used in combination with markers for basal cells and less specific indicators of prostate cancer such as P504S (AMACR; racemase). This preserves precious biopsy tissue and reduces labor and reagent costs dramatically. More importantly, this Multiplex IHC is clinically useful in the differentiation of prostate cancer from PIN. According to Sesterhenn, the addition of basal cell markers are particularly useful in differentiation of atrophy from prostate cancer. Also, elucidation of PIN from branching PIN is possible since marking basal cells allows the pathologist to visualize if extensions (smaller glands) are connected to and part of the PIN gland, or if they are separate and therefore indicative of invasive disease.
“ERG is a phenomenal breakthrough in the specificity of prostate cancer diagnosis; having an antibody for IHC, as opposed to a more complex FISH method to detect ERG gene translocation greatly simplifies this technology. All of this is the holy grail of prostate cancer detection,” said David Tacha, PhD, CSO and vice president of research and development, BioCare Medical LLC.
Mark Cross, Sr, is director of sales and marketing, and Rhonda Henshall-Powell, PhD, is director of marketing and education, for BioCare Medical.
References
- Furusato B, Tan SH, Young D, et al. ERG oncoprotein expression in prostate cancer: clonal progression of ERG-positive tumor cells and potential for ERG-based stratification. Prostate Cancer Prostatic Dis. 2010;13(3):228-237. Epub 2010 Jun 29.
- Patel AR, Jones JS, Rabets J, DeOreo G, Zippe CD. Parasagittal biopsies add minimal information in repeat saturation prostate biopsy. Urology. 2004;63(1):87-89.
- Haas GP, Delongchamps NB, Jones RF, et al. Needle biopsies on autopsy prostates: Sensitivity of cancer detection based on true prevalence. J Natl Cancer Inst. 2007;99(19):1484-1489. Epub 2007 Sep 25.
- Park K, Tomlins SA, Mudaliar KM, et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia. 2010;12(7):590-598.