 |
Regulations are not built in a single day, and neither are the compliance efforts that ensure requirements are met. In fact, regulatory and compliance programs are never really finished. If inflexible, they, too, would become as obsolete as the early technologies and techniques the first rules regulated.
Instead, regulatory agencies adjust continually (though not always rapidly) as new research is published and technology is developed. Evidence that supports new methods and modalities as safe, effective, and often improved provides the material used to develop these guidelines.
 |
 |
 |
| Steward Macis of Antek HealthWare | Curt Johnson of Orchard Software | Dave Hunt of C2C |
Unfortunately, the constantly morphing regulations—though driven by lofty goals—can be confusing and challenging for laboratory officers focused solely on compliance, let alone those managers trying to juggle too many balls. Regulations impact procedures, staffing, quality control, safety, and sanitation, and they are created by multiple organizations, from the health care institution itself through industry associations and regional governments to federal agencies. Measurements must be regularly taken, and documentation must show everything.
If steps are missed, mistakes are made, or documentation is missing, laboratories will pay the price, which can range from warnings and fines to lab closings and patient fatalities. There is, therefore, very little room for error.
 |
| Antek’s LabMail feature helps meets the OIG requirements for effective lines of communication between the compliance officer and lab staff. |
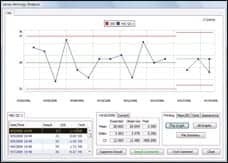 |
| Orchard’s Harvest LIS features a quality control module that allows users to view reports and graphs at any time. |
Subsequently, compliance is an excellent area for technology to assist. The less manual a process, the less likely there is error to be introduced—particularly human error. Automation, software, and service partners can help to ensure that rules are updated and the information is recorded.
Would it be helpful to make sure a test and the information gathered are appropriate for the patient before running it—instantly? There’s a program for that. Do you want all e-mails sent from the laboratory with results to be saved for 5 years? There is a program to help with that. Do you want to automatically record your refrigerator temperatures and have them at easy recall? There is a program to help with that. We know because CLP took a look at the market and talked to some of the experts about available offerings.
Antek Looks at OIG Requirements
“It can be very challenging for laboratories to keep pace with the ever-changing regulatory environment. There are several agencies and organizations that drive clinical laboratory compliance, but they all stem from the Department of Health and Human Services Office of Inspector General (OIG),” says Steward Macis, vice president of products and support services for Antek HealthWare LLC, in Reisterstown, Md.
OIG’s Compliance Program Guidance for Clinical Laboratories, published in 1988, lays out the global perspective and broad guidelines for ethical and lawful conduct for clinical laboratories. Associations and other laws, such as the College of American Pathologists (CAP), COLA, and Clinical Laboratory Improvement Amendments (CLIA), then drill down to more specific details.
Macis sees three existing areas where labs experience the “largest headaches” when dealing with OIG compliance: keeping up with the changes in HIPAA, Advanced Beneficiary Notice (ABN), and Medicare reimbursements. Antek has designed some of its services and product features to help with these challenges.
The company’s HIPAA tool kit includes a summary of the law, sample forms, an outline of new procedures, and checklists. “Our goal is to protect the privacy and security of individually identifiable health information and our clients’ ability to use our services,” Macis says.
To handle the new ABN procedures, new forms have been implemented in LabDAQ, the company’s laboratory information system (LIS). Information about the change was publicized through newsletters, account managers, online user groups, and e-mail. Software updates include appropriate modifications.
Reimbursement changes are also regularly updated through software. The system’s mapping files can be updated quarterly, semiannually, or annually. Antek partners with CodeMap, Barrington Ill, which specializes in Medicare CPT and diagnosis code validation. “We have created … a utility that allows users to associate ICD-9 codes with CPT codes to ensure that reimbursement will occur,” Macis says.
Other LabDAQ features cover additional compliance areas, including auditing and staff evaluation, monitoring reports and data mining capabilities, standing orders, proficiency testing, test utilization, and e-mail tools. “The evolution of compliance has put us in a position to now routinely perform quality assessments on the three phases that drive the clinical laboratory today, which are preanalytical, analytical, and postanalytical testing. Looking ahead, laboratory compliance will have to address how new technologies and innovations will affect these three phases,” Macis says.
Orchard Considers the Three Phases
Curt Johnson, vice president of sales and marketing for Orchard Software, Carmel, Ind, asks what tools an LIS can provide users to improve each phase of laboratory testing. Orchard also is continually updating software; it’s most recent release, Harvest LIS version 8.0, featured more than 225 enhancements and additional features. “Typically, 80 to 85 percent [of modifications] are suggested by customers,” Johnson says.
In the preanalytical stage, the LIS offers positive patient identification tools and a rules engine to assist with test appropriateness and labeling. Because each lab has its own unique rules, the system has been designed to be flexible.
Rules are established through all three stages and include auto-approval of patient results, reflexing additional testing, automatic comments, clinical follow-up recommendations, and order splitting. Special modules address microbiology and pathology; users can write microbiology threshold rules or have technical instructions automatically displayed.
The rules-based technology helps to increase productivity, maintain consistency, and simplify training. Rules can be based on numerous data points, including test results, delta violations, providers, locations, patients, order priorities, and patient ages.
The quality control module features graphs, patient result approval rules, and immediate access to quality control records. “Do you have the ability to change the mean? Can you keep historical means? How are you handling linearity, calibrations, and maintenance? It all falls in the analytical phase, and we have tools built in to enhance the experience of users,” Johnson says.
The system also has tools to handle the postanalytical phase as well, where documentation and data mining are key to compliance. “When you have a critical result, how do you document it? How are they delivered? Can you amend a report? How is that documented and communicated? When you have exceptions to the rules, how well can you document that?” Johnson asks.
Everything is built into the system, and users decide which features and tools suit their laboratories best. The capabilities are intended to help laboratories eliminate manual logs and enjoy the resulting benefits of immediate electronic access to information. “Tools have to integrate into your workflow and offer a benefit over how you are doing it now,” Johnson says.
 |
| OneSource Laboratory Services provides a range of qualification solutions from traditional paper-based qualification to an advanced metrology-based system. |
C2C Controls E-mail
C2C Systems Inc, located in Springfield, Mass, offers a tool to help organize and store e-mail messages. HIPAA requires hospitals and doctors to keep billing records and authorization forms for 6 years, and other patient data for the life of the patient.
“One of the dangers with e-mail is that an individual e-mail can be taken out of context, so you do need to maintain strings of e-mail,” says C2C CEO Dave Hunt. But storing and accessing old e-mails can prove challenging. Even if the rules are specified to minimize the number of e-mails saved, consistent application of the policy across the organization is unlikely.
“If you try to be selective about what you keep and don’t keep—even if it goes out as a company-wide memo—people will have their own different interpretations, and the rules may get forgotten or modified over time,” Hunt says.
C2C offers an e-mail archiving solution that stores e-mails according to user-set rules in a compressed format, which saves space. The information, however, is still easily searchable and retrievable. “How much does it cost an organization to look for an e-mail if they have a request to prove something?” asks Hunt, who notes that preparation for judicial requests can run into tens of thousands of dollars. Archive One permits immediate collection of relevant e-mail. It also incorporates security measures that prevent access to data by inappropriate personnel.
Each institution can set the rules that determine which e-mails are kept, which are deleted, and who has access according to its needs and the regulations it must meet. Compliance officers often determine the policies, but legal counsel may have a say, too.
“We allow them to create a rule that says if an e-mail has this particular set of words or is from a certain company or person, then put it into an archive and keep it for 5 years,” Hunt says.
Those words can be found in the body of the e-mail or any attachments. In fact, rules can be written to save particular attachments, such as all digital slides. So appropriate attachments are saved, but if used in multiple e-mails, only one copy is stored on the archive. “We employ three different methods to allow storage savings,” Hunt says.
 |
Storage savings can be significant with the implementation of C2C’s system. “We can reduce the storage load on [an institution’s] primary server by 90 percent and implement a secondary storage platform, which is cheaper to manage,” Hunt says.
Isensix Arms Labs with Equipment Monitoring Tools
San Diego-based Isensix Inc also offers a solution designed to make managing a compliance program easier. Its Advanced Remote Monitoring Systems (ARMS) monitors the key operating parameters of storage appliances, a process that in many laboratories is still handled manually.
Typically, staff collect data from the various storage units and record them on daily log sheets or they collect expired rotary charts. The process is an inefficient use of laboratorian time and is prone to human error, and the costs of inaccurate record keeping can be significant. The loss of product and samples, and the ramifications of noncompliance, can be expensive.
ARMS provides a 24/7, wireless, real-time solution. Wireless sensors monitor equipment and transmit the data wirelessly to access points, which in turn send the data via the institution’s local area network to the system host. The ARMS system is accessible via a Web browser on any networked PC, as well as centrally at a dedicated workstation that provides easy access to the data and alerts staff when equipment metrics fall outside the parameters. Urgent notifications can alert appropriate personnel using visual and audio cues as well as e-mail, paging, and voice alerts.
Different sensors take different measurements. Isensix’s offerings can monitor temperature, door access and security, humidity, carbon dioxide and oxygen, pressure and differential pressure, airflow, BacT positive result detections, horizontal motion, and 4-20 mA customized solutions. If a measurement indicates a problem, staff is automatically notified according to the rules. Everything is documented, stored, and easily accessible.
 |
| Paul Smith of PerkinElmer |
Installation is quick, and on-site training is available. The system fully complies with the FDA 21CFR Part 11 electronic records requirement, and its validation, calibration, and documentation meet the requirements of The Joint Commission, CAP, FDA, and AABB (formerly known as the American Association of Blood Banks).
One goal of the system is to reduce unnecessary citations for noncompliance and revisits from government agencies. Another is to permit staff to use their time on higher-level tasks rather than monitoring equipment.
PerkinElmer Offers OneSource
PerkinElmer Inc, Waltham, Mass, also aims to let laboratorians focus on clinical analysis instead of managing the administration of a compliance program. OneSource Laboratory solutions assist with instrument repair, preventive maintenance, and qualification of equipment across the entire laboratory. Methods range from traditional paper-based qualification to metrology-based solutions that utilize patented hardware and propriety 21CFR part 11 technically compliant software.
The services directly maximize equipment uptime and indirectly increase laboratory productivity. All planned and preventive maintenance requirements are met, and PerkinElmer proactively monitors compliance trends and updates its service to meet changes. “Meeting changing regulatory demands is an ongoing challenge and can be a drain on resources,” says Paul Smith, validation manager for PerkinElmer’s OneSource Laboratory Services. Having a provider who tracks these changes can help.
PerkinElmer monitors all regulatory agencies and acts, from CLIA to ISO 15189. “Implementing the requirements of ISO 15189 can be daunting for laboratory managers not experienced in monitoring performance of laboratory equipment. Instrument qualification processes based on defendable scientific rationale are essential. However, for laboratories using qualification services from a range of suppliers, this rationale can be variable and difficult to justify,” Smith says.
Using one provider can overcome these obstacles and can save money through the consolidation of costs. “Measurable benefits are typically observed within the first year of adopting a consolidated compliance and instrument qualification approach with OneSource Laboratory Services,” Smith says.

To stay up to speed on compliance issues, bookmark our website. |
An integrated approach can ensure consistent application of rules to documentation and other areas, which helps to ensure audit readiness. PerkinElmer’s Installation and Operational Qualification produces a thorough audit trail, and Instrument Performance Verification Services provides verification certificates and compliance stickers on-site.
“Compliance is not something that is achieved on a particular day. It is something that all laboratory managers have to continually strive toward,” Smith says. By using available technology and partners, laboratories can reduce operational costs, decrease errors and failures, experience faster audits, and ultimately, focus on clinical analysis.
Renee Diiulio is a contributing writer to CLP.



