Abbott, Abbott Park, Ill, has agreed to supply its proprietary PathVysion human epidermal growth factor receptor 2 (HER2) DNA fluorescence in situ hybridization (FISH) probe kits to Angle plc, Guildford, United Kingdom. The kits will be provided in the form of a research grant for use in Angle’s ANG-002 study of FISH analysis for circulating tumor cells (CTCs).
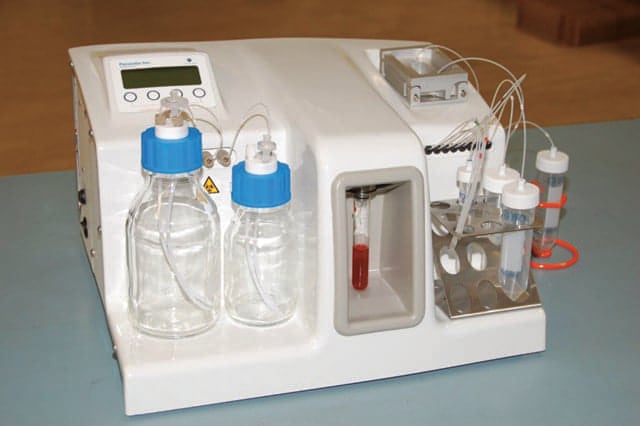
FISH analysis is used to investigate cancer cells discovered during a solid-tissue biopsy in order to help select treatment, and is one of the exploratory end-points for Angle’s study of metastatic breast cancer. Angle is seeking to demonstrate that CTCs can be harvested from the blood of metastatic breast cancer patients using the company’s Parsortix system, and that the harvested CTCs can then be subjected to FISH analysis to determine patients’ HER2 status.
Abbott’s PathVysion HER2 DNA FISH probe kit is used for genomic disease management. Physicians use PathVysion HER2 results to help determine which patients may be the best candidates for personalized therapy. Specifically, PathVysion is designed to determine the status of a patient’s HER2 gene, identifying which patients are HER2 positive.
About one in every five breast cancer patients has a positive HER2 result, meaning that they are more likely to respond to Herceptin therapy. Herceptin can help control the growth of cancer cells that contain high amounts of HER2. Herceptin works by blocking the effects of HER2 and encouraging the immune system to attack and kill the cancer cells.
“Abbott is pleased to collaborate with Angle in this important evaluation of PathVysion in liquid biopsy specimens,” says Kathryn B. Becker, PhD, franchise director of oncology and companion diagnostics at Abbott. “The PathVysion HER2 DNA FISH probe kit is reliable and accurate in tissue biopsy samples, and the Parsortix system may unlock the potential for PathVysion use in a simple blood test. We look forward to the outcomes of the study and the potential opportunity to further collaborate with Angle in combining FISH and liquid biopsy in other areas.”
Abbott’s PathVysion test was released in 1998, and was the first gene-based test approved by FDA for determining HER2 status. A positive PathVysion HER2 result in Parsortix-harvested CTCs would demonstrate the feasibility of the assay for use in breast cancer liquid biopsies in metastatic breast cancer patients.
“We hope to be able to work with Abbott to extend PathVysion use into routine blood test analysis as an important downstream application of the Parsortix system in breast cancer,” says Andrew Newland, founder and chief executive of Angle. “Use of Angle’s Parsortix system would for the first time enable established tissue biopsy techniques to be used for breast cancer on cancer cells obtained from a simple blood test, protecting patients from invasive procedures and improving their care whilst at the same time reducing healthcare costs.”