The Atomo HIV self-test from Atomo Diagnostics, Sydney, Australia, is now eligible for procurement by organizations entitled to access Global Fund and Unitaid resources.
The integrated human immunodeficiency virus (HIV) self-test device has been approved by the expert review panel for diagnostics (ERP-D) of the World Health Organization (WHO), using a special process created by Unitaid and the Global Fund to expedite access to innovative diagnostic products in advance of approvals through the WHO prequalification process.
The test is available for organizations accessing funding for HIV self-test pilot programs that empower individuals to take control of their own health.
The test was recently granted a CE mark, permitting Atomo to market the product in Europe. The ERP-D approval will facilitate further expansion and commercial rollout of the product in low- and middle-income countries.
“We are delighted that the ERP-D has included the Atomo HIV self-test on the Global Fund procurement list,” says John Kelly, founder and CEO of Atomo Diagnostics. “This will enable further expansion of HIV self-testing beyond the private sector and into the growing global health sector, and will support programs aimed at ensuring early diagnosis of HIV.”
When used by untrained self-test users in the field, the Atomo HIV self-test, which is designed to be used at home, demonstrated 100% concordance to laboratory results in independent studies.
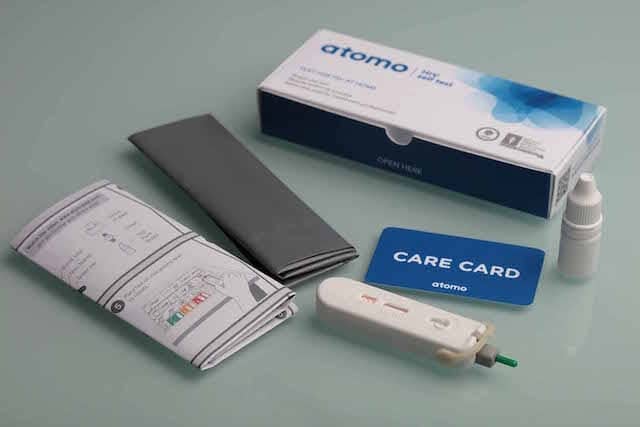
The third-generation HIV test strip, used in a handheld device, incorporates a sterile safety lancet and unique blood collection and delivery system. The test is performed by delivering a small drop of capillary blood from a fingerstick onto the test strip, and then applying drops of chase buffer. Within minutes, a control line will become visible on the test strip to indicate that the test has worked. A second line—the test line—will only become visible if the applied sample contains antibodies to HIV.
For more information, visit Atomo Diagnostics.





I would like to distribute your products in Chile.
Let me know if you have availability to do this.Thank you very much for your attention
Hernan Fuenzalida
It has been awaited in the pipeline. i hope the sensitivity and specificity is high.