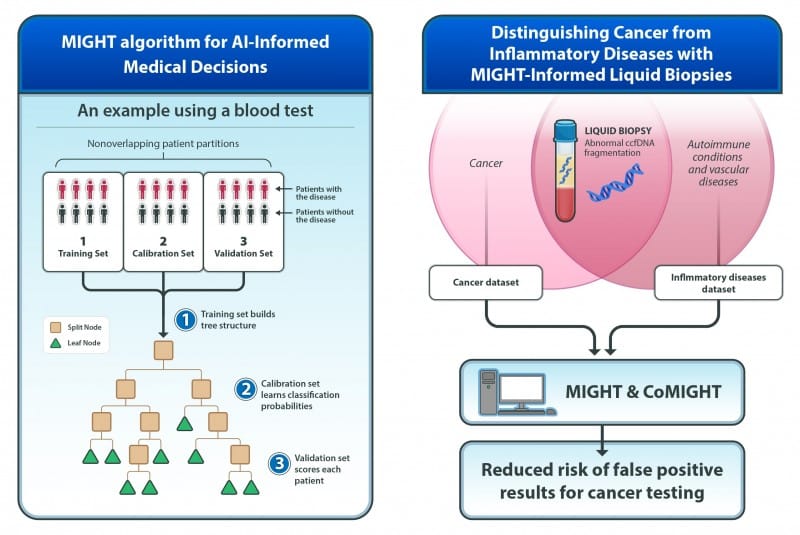
MIGHT algorithm addresses key challenges in clinical AI implementation by improving accuracy and reducing false positives in liquid biopsy testing.
Researchers at Johns Hopkins have developed a new artificial intelligence (AI) method that significantly improves the reliability and accuracy of AI tools for clinical applications, with particular promise for early cancer detection through liquid biopsy testing.
The method, called MIGHT (Multidimensional Informed Generalized Hypothesis Testing), was designed to meet the high confidence levels required for AI tools used in clinical decision-making. Two studies describing the technology and its applications were published in the Proceedings of the National Academy of Sciences.
MIGHT uses tens of thousands of decision trees to fine-tune itself with real data and check accuracy across different data subsets. The algorithm is particularly effective for analyzing biomedical datasets with many variables but relatively few patient samples, a common challenge where traditional AI models often struggle.
In testing with patient data, MIGHT consistently outperformed other AI methods in both sensitivity and consistency. When applied to blood samples from 1,000 individuals—352 patients with advanced cancers and 648 individuals without cancer—the algorithm achieved 72% sensitivity at 98% specificity using aneuploidy-based features.
“MIGHT gives us a powerful way to measure uncertainty and increase reliability, especially in situations where sample sizes are limited but data complexity is high,” says Joshua Vogelstein, PhD, associate professor of biomedical engineering and lead investigator, in a release.
Addressing False Positives in Liquid Biopsy
A companion study revealed that circulating cell-free DNA fragmentation patterns previously thought to be cancer-specific also appear in patients with autoimmune diseases like lupus and systemic sclerosis, as well as vascular diseases including venous thromboembolism.
The research found that inflammation—rather than cancer itself—appears responsible for these fragmentation signals, complicating efforts to use ccfDNA fragmentation as a cancer-specific biomarker.
“Our main goal was to further investigate the biological mechanisms responsible for fragmentation signatures that have previously been thought to be specific for cancer,” says Samuel Curtis, PhD, postdoctoral fellow in the Ludwig Center, in a release. “As the field moves to more complex biomarkers, understanding the underlying biological mechanisms leading to the results are critical to their interpretation, particularly to avoid false positive results.”
To address this challenge, researchers incorporated inflammation data into MIGHT’s training, which reduced but did not completely eliminate false-positive results from non-cancerous diseases.
Clinical Implementation Challenges
The researchers also published a related editorial in Cancer Discovery identifying eight key barriers to bringing AI into routine clinical care. These include unrealistic expectations for AI perfection, the need to present results as probabilities rather than binary answers, ensuring reproducibility, training on diverse populations, and avoiding over-reliance on computer-generated recommendations.
The team extended MIGHT to create CoMIGHT, a companion algorithm that determines whether combining multiple variable sets could improve cancer detection. Testing on 125 patients each with early-stage breast and pancreatic cancers, along with 500 controls, showed that early-stage breast cancer detection might benefit from combining multiple biological signals.
“Trust in the result is essential, and now that there is a reliable, quantitative tool in MIGHT, we and other researchers can use it and focus our efforts on studying more patients and adding statistically meaningful features to our tests for earlier cancer detection,” says Bert Vogelstein, MD, Clayton Professor of Oncology and study co-leader, in a release.
The researchers emphasize that AI results should complement rather than replace clinical judgment, and that further clinical trials and validation are necessary before such tests can be used clinically.
Both MIGHT and CoMIGHT are now publicly available at treeple.ai. The studies were supported by multiple National Institutes of Health grants and involved collaborators from institutions in the US, Vietnam, Australia, Canada, and the Netherlands.
Photo Credit: Elizabeth Cooke
Photo caption: MIGHT algorithm for AI-informed medical decisions and MIGHT-informed liquid biopsies for distinguishing cancer from inflammatory diseases