Research across nine cancer types found the assay identified molecular progression over five months before imaging in advanced tumors.
A new study published in npj Precision Oncology demonstrates that Personalis Inc’s ultrasensitive molecular residual disease assay can detect immunotherapy response in advanced cancer patients weeks before conventional monitoring methods.
The research, led by oncology researchers at UC San Diego Moores Cancer Center, analyzed 39 patients with advanced solid tumors across nine different cancer types treated with immune checkpoint inhibitors alone or in combination with other therapies.
The NeXT Personal test uses a personalized approach that tracks up to 1,800 tumor-specific variants unique to each patient’s tumor to detect circulating tumor DNA (ctDNA) at ultrasensitive levels from blood samples.
Early Detection Capabilities
The study found that molecular response, defined by ctDNA dynamics, was detectable at a median of 23 days after starting immunotherapy. Patients achieving an early molecular response had significantly longer progression-free survival compared to those who did not.
For patients whose disease progressed, the NeXT Personal test identified molecular progression a median of 161 days before imaging detection. The research also showed that patients who achieved molecular complete response, defined as ctDNA clearance, had seven times higher overall survival than patients who did not achieve ctDNA clearance.
Even in advanced tumors where ctDNA shedding can be higher, 33% of positive ctDNA detections occurred in the ultrasensitive range below 100 parts per million. These detections could potentially be missed with less sensitive tests, according to the researchers.
“We continue to expand the clinical evidence that NeXT Personal can be used to monitor therapy response in advanced cancer patients on immunotherapy,” says Richard Chen, MD, MS, chief medical officer and executive vice president of R&D at Personalis, in a release. “This pan-cancer study builds on our recent publication in Clinical Cancer Research, similarly showing the impact of ultrasensitive ctDNA testing in late-stage cancers.”
Addressing Clinical Need
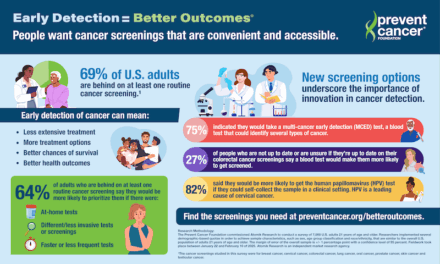
The research addresses a clinical challenge in cancer care, as only 10% to 40% of patients achieve durable benefit from immunotherapy, making it critical to monitor patient response to treatment, according to a release from Personalis.
“With immunotherapy, an important pillar of cancer treatment in advanced cancer patients, the need for better tools to evaluate patient response is increasingly important,” says Chen in a release. “These findings show how NeXT Personal and ultrasensitive ctDNA testing can potentially play an important role in impacting care across a broad spectrum of solid tumors.”
ID 27706957 © Mrfocoz | Dreamstime.com