DRI Ecstasy Assay
Rapid analytical test

The new DRI ecstasy assay from Beckman Coulter Inc, Fullerton, Calif, provides a rapid analytical test intended for the qualitative or semiquantitative determination of ecstasy drugs in human urine, at a cutoff level of 500 ng/mL. It uses a specific antibody that can detect ecstasy drugs in urine with minimal cross-reactivity to various amphetamine compounds. When run on the company’s UniCel DxC or Synchron CX/LX Clinical Systems, a qualitative, interpretive report automatically compares patient results to the cutoff value and determines positive or negative, eliminating the need for manual interpretation. An estimate of drug concentration in the samples can be obtained using the semiquantitative method that uses five calibrator levels. Reagents and calibrators for this assay are liquid and ready to use. The product is manufactured by Thermo Fisher Scientific and distributed by Beckman Coulter as part of its menu of automated chemistry assays. Used by clinical labs.
Beckman Coulter Inc
(800) 352-3433
www.beckmancoulter.com
EZ Split Key Cup II
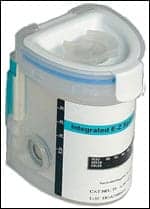
Offers a hinged lid with an audible snap and an extra seal
Innovacon Inc, San Diego, introduces the EZ Split Key Cup II, which offers the same test format as the original cups but includes a new hinged lid that produces an audible snap and extra seal to ensure sample retention during shipping. It provides key storage in the lid. The all-inclusive drug test cup combines a drug screen and adulteration strip in a one-step test. The design eliminates contact with urine, automatically splits the specimen for sample confirmation, and the test cup can be photocopied. The user-friendly cup is available in many drug combinations. CLIA-waived configurations are also available. The company’s rapid test products are available in generic OEM packaging or custom formats, including customized devices, custom packaging, and custom labeling. Used for workplace testing, by physician’s office labs, by substance abuse rehab facilities, and in criminal justice markets.
Innovacon Inc
(858) 535-2039
www.innovaconinc.com
OratectPlus Oral Fluid Drug and Alcohol Screen Device
One device detects drugs and alcohol

Branan Medical Corp, Irvine, Calif, combines drug and alcohol oral fluid testing in a single device with the new OratectPlus. The on-site saliva screen test allows test administrators to obtain rapid results minus the concern of handling a urine specimen. The device simultaneously tests for six drugs, including marijuana, cocaine, opiates, amphetamine, methamphetamine/ecstasy, and either PCP or benzodiazepines plus alcohol. The reliability of an oral-fluid test is comparable to urinalysis testing for the most commonly abused drugs. The product has a collection pad on one end, two test panels in the middle for the drug results, and a separate pad for the alcohol result. The test protocol entails swabbing the collection pad inside the mouth until blue lines in the test panels start to flow, indicating a sufficient amount of saliva has been collected. A red line corresponding to a specific drug on the panel indicates a negative result, while the absence of a red line indicates a presumptive positive result. A color change from white to green, blue, or gray indicates a presumptive positive alcohol result. With a presumptive positive result, the collection pad is detached from the device and sent to a SAMHSA lab for further testing, allowing confirmation of the on-site result without drawing another sample. Used by human resources managers and criminal justice professionals.
Branan Medical Corp
(866) 468-3287
www.brananmedical.com
Synchron DAT and Olympus DAT
Test for a myriad of substances

Carolina Chemistries, Brea, Calif, offers new drugs-of-abuse tests packaged in bar-coded cartridges for Beckman Synchron CX, LX, and DXc chemistry analyzers. The reagents are liquid, ready-to-use cartridges, with 250 tests per cartridge. Synchron DAT and Olympus DAT are stable on board for 60 days and require calibration once every 14 days. The company offers bar-coded drugs-of-abuse reagents specifically designed for use on Olympus AU400, AU640, AU2700, and AU 5400 chemistry analyzers. Each kit contains 1,200 tests, is stable on board for 60 days, and has calibration stability of 14 days. The reagents are accompanied by multianalyte calibrators and controls. Available tests include alcohol, cocaine metabolite, amphetamines, methadone, opiate 300, cannabinoid, phencyclidine, propoxyphene, benzodiazepine, barbiturates, and oxycodone. Used by reference labs and hospitals.
Carolina Chemistries
(714) 529-1616
www.carolinachemistries.com
OnTrak TesTcard 9

In vitro diagnostic
The OnTrak TesTcard 9 from Varian Inc, Palo Alto, Calif, is a rapid in vitro diagnostic Point of Care (POC) device primarily used in hospitals to determine if patients entering the emergency department could be under the influence of drugs. It provides a qualitative screen for the presence of amphetamines, barbiturates, benzodiazepines, cocaine, methamphetamines, morphine, phencyclidine (PCP), THC, and tricyclic antidepressants (TCA) in urine. The device is a single-step test that requires no reagent mixing, timing, incubation, or special handling. Results for all nine drugs are available in 3 to 5 minutes. Test results are stable for up to 6 hours, which enables batch processing for the POC device. It is used by emergency departments, labs, and drug-treatment facilities.
Varian Inc
(800) 737-9667
www.varian-onsite.com
Capillarys CDT Assay
Biomarker

The automated carbohydrate-deficient transferrin (CDT) assay from Sebia Electrophoresis, Norcross, Ga, is a specific biomarker for detection of chronic ethanol consumption. It is performed via capillary electrophoresis and is a completely automated, stand-alone method that has received FDA clearance. The assay is suitable for screening and confirmation analysis due to its ability to visualize the total transferrin isoform pattern and to detect genetic transferrin variants. It automatically calculates percent CDT for each sample. Chronic alcohol (ethanol) use increases the concentrations of the CDT isoforms. The consumption of approximately 60 grams (or four to five drinks) of ethanol per day for a period of 7 to 10 consecutive days is required to cause an increase in the serum CDT fraction. Because CDT reflects alcohol intake during the 1 to 2 weeks prior to blood collection, and CDT levels return to normal in about 14 days, the test is suited for monitoring relapse and abstinence in an alcohol treatment setting. There is a trend toward investigating the utility of alcohol biomarkers as disease risk indicators in surgical and trauma settings. One study concluded that, due to the medical complications caused by excessive drinking, for high-risk surgical and trauma patients, a detailed admission work-up is recommended, including an alcoholism-related questionnaire and preoperative CDT, GGT, and MCV testing.
Sebia Electrophoresis
(800) 835-6497
www.sebia-usa.com
Tox STATus
One-step process

Nanogen Inc, San Diego, offers a new product that accurately identifies a wide range of drugs of abuse in a cost-effective configuration. Featuring a comprehensive test menu, Tox STATus detects up to 10 drugs of abuse with a single test, helping hospitals and urgent care facilities to maximize reimbursement claims and contain costs. The device eliminates cumbersome steps during the testing process, which helps meet the demands of urgent care environments. Positive results can be read in 5 minutes, and lab-correlated results are ready in 15 minutes or less. It can be used as a tool that helps assess and treat patients with complications due to drug overdose. The product can be stored at room temperature for up to 18 months, requires low sample volume, and accommodates multiple drug combinations. It has been cleared by the FDA. Test results are stable for up to 4 hours, and the product provides 99% correlation to GC/MS at 95% confidence level. A control line ensures an accurate reading. An optional i-Lynx data-capture device is offered that is an HL7 POCT1-A compliant interface. Used by emergency departments, emergency medical technicians, the military, and correctional facilities, and in the workplace.
Nanogen Inc
(888) 354-3278
www.nanogen.com
Urine Screen Controls
Include codeine, LSD

Urine screen controls from Quantimetrix Corp, Redondo Beach, Calif, monitor the performance of Abuscreen ONLINE, AxSYM, EMIT, Triage, and other drugs-of-abuse screening methods. Newly added to the drugs-of-abuse control are codeine, LSD, MDA, MDMA, and MDE. The product is liquid, human urine-based, and has a 3-year unopened and open-vial stability when stored at 2°C to 8°C (from date of manufacture). The control meets current revised guidelines established by the Substance Abuse and Mental Health Services Administration. Level 1 is drug-free urine. Level 2 has drug analytes below screening cutoff levels (negative threshold screen). Level 3 is designed to contain drug analytes above screening cutoff levels (positive screen).
Quantimetrix Corp
(800) 624-8380
www.4qc.com


