Faster, Lower-Cost Amino Acids Analysis
Full analysis of 46 amino acids in 90 minutes

Biochrom Ltd, Cambridge, United Kingdom (with US headquarters in Holliston, Mass), a leading manufacturer of dedicated amino acid analyzers, has released a new accelerated method for amino acid analysis in physiological samples such as plasma, urine, and cerebrospinal fluid. Results are delivered reportedly 30% faster than the high-performance method, with a full analysis of 46 amino acids achieved in just 90 minutes. Optimum peak resolution, accuracy, and sensitivity are maintained. Users of the accelerated method also benefit from a 25% reduction in the cost of analysis due to lower reagent consumption. The new accelerated method is included as standard with the new Biochrom 30+ Amino Acid Analyzer, and is also compatible with the Biochrom 30 amino acid analyzer.
Biochrom Ltd
(877) 246-2476
www.biochrom.co.uk
CrystalQuick X Microplate
Suited for in situ x-ray, UV analysis of protein crystals

Greiner Bio-One, Frickenhausen, Germany (US offices in Monroe, NC), an international technology partner for the diagnostic and pharmaceutical industry, presents the 96 Well CrystalQuick X Microplate, a first-of-its-kind microplate to be specifically tailored to the requirements of x-ray structure analysis of protein crystals directly in the plate. It is also optimal for UV screening of crystals. This new microplate dramatically improves the results of in situ crystal analyses. Its open geometry allows data to be collected directly in the plate within an angular range of up to 80°. An ultrathin-walled well bottom minimizes background scattering significantly. Thanks to its excellent optical properties, the new microplate is ideal for bright-light microscopy as well as applications involving polarized and UV light. With two flat-bottom crystalization wells per reservoir, CrystalQuick X makes it possible to test 192 sitting drop samples per plate. The plate features alphanumeric well coding and an additional scale (0.1 x 0.5 mm), which enables the quick estimation of crystal size. Its footprint conforms with the ANSI/SBS 1-2004 standard. The plate is therefore compatible with all conventional automatic systems.
Greiner Bio-One North America
(704) 261-7800
www.us.gbo.com
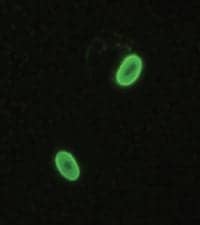
New Giardia Monoclonal Antibodies
Reactive with the cyst form of the organism
ViroStat Inc, Portland, Me, a primary manufacturer of infectious disease antigens and antibodies, has introduced several new monoclonal antibodies to Giardia. These are reactive with the cyst form of the organism and target the GS65 antigen. Giardia is the leading cause of parasitic gastrointestinal disease in the United States. It occurs most commonly following the ingestion of water contaminated with the Giardia lamblia organism. Other specialties from Virostat include high-affinity antibodies to Flu A, Flu B, RSV, and Strep A for use in rapid lateral flow devices, as well as antibodies to food-borne pathogens and toxins. Also from ViroStat are many specificities to HCV, HBV, CMV, and EBV for use in antiviral HTS assays.
ViroStat Inc
(207) 856-6620
www.virostat-inc.com
Rapid CDI Infection Test Kit
Results in 20 minutes or less
From Sekisui Diagnostics (formerly Genzyme Diagnostics), San Diego, comes the company’s OSOM C. difficile Toxin A/B, a rapid test to aid in the diagnosis of C. difficile Infection. Just four simple steps, walk away, and read the results in 20 minutes or less. The test has the ability to be run batch or STAT. An accurate, easy to read, two-color dipstick provides clear objective results, and as an added value, each kit contains two additional test sticks for external QC. The kit contains all materials necessary to run the test. Online training is available anytime at www.osomtraining.com.
Sekisui Diagnostics
(800) 332-1042
www.sekisuidiagnostics.com
New Immunosuppressant Immunoassay
Thermo’s QMS Everolimus receives 510(k) FDA clearance

Thermo Fisher Scientific Inc, Fremont, Calif, announced its new QMS Everolimus Reagent kit, the newest assay in the group of immunosuppressant drug monitoring products. The QMS Everolimus Reagent kit, which rounds out the company’s full array of immunosuppressant immunoassays that includes Cyclosporine, Tacrolimus, and Mycophenolic Acid, was reportedly the first to receive FDA clearance for monitoring the appropriate blood levels of everolimus, a drug that helps prevent rejection in kidney transplant patients. This ready-to-use liquid QMS Everolimus assay is used as part of therapeutic drug monitoring, an integral part in organ transplant recipient treatment programs. Based on the Thermo Scientific Quantitative Microsphere Systems (QMS), a homogeneous particle-enhanced turbidimetric technology, the Thermo Scientific QMS Everolimus assay is used on automated clinical chemistry analyzers for quantitative determination of everolimus in human whole blood, the active ingredient in the Novartis immunosuppressant drug—trade name Zortress. Strategies for immunosuppressive treatment have focused on preventing T lymphocyte cell activation and/or proliferation. Everolimus acts as a proliferation inhibitor. Thermo Scientific QMS Everolimus Calibrator and Control sets are also available for use with the QMS Everolimus Assay. The calibrator kit contains six different calibrators to complete a full six-point calibration, as part of the test procedure; and the QMS Everolimus Control Set contains three levels.
Thermo Fisher Scientific Inc
(800) 232-3342
www.thermoscientific.com

TruTip DNA Kits
Delivers PCR-ready DNA five times faster
Extracting and purifying human genomic DNA for genetic testing, pharmacogenomics, and forensic applications has now been revolutionized with Frederick, Md-based Akonni Diagnostics’ TruTip. Used in conjunction with an Eppendorf epMotion automated pipetting station, TruTip delivers inhibitor-free, PCR-ready DNA up to five times faster than gold standard spin columns, and all with the simple push of a button—or perform up to eight ultrarapid manual extractions with TruTip and a Rainin EDP 3Plus single- or multichannel pipette, in as little as 4 minutes.
Akonni Diagnostics
(301) 698-0101
www.akonni.com


