Molecular diagnostics company Seegene today announced the development of a commercialized PCR assay that applies “3 Ct” technology.
The Allplex HPV HR Detection assay was recently showcased at the 2022 European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Lisbon, Portugal.
In a polymerase chain reaction (PCR), the cycle threshold (Ct) value is used to quantify the concentration of a viral DNA sequence. Due to technological limitations, the conventional real-time PCR technique finds the Ct value of one target in one channel.
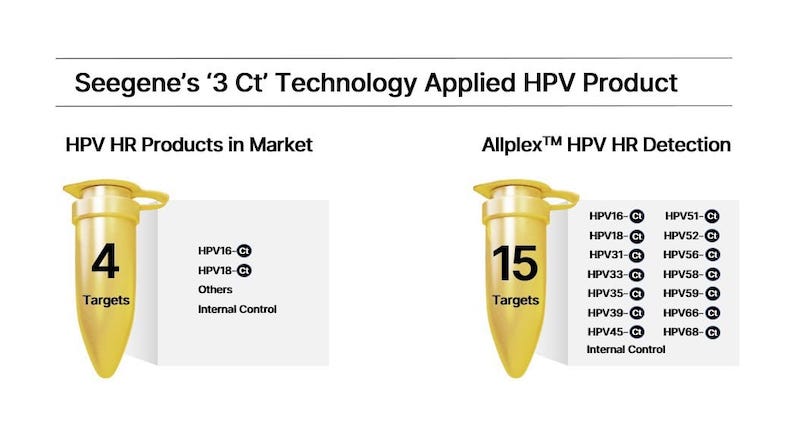
But Seegene’s “3 Ct” technology can provide the Ct value of three targets in one channel without compromising sensitivity and specificity. The new development combines 19 different patented technologies, including DPO, TOCE, and MuDT. Using five channels in a single tube, Seegene can provide quantitative data for a total of 15 targets.
The company plans to apply “3 Ct” technology to its entire product line-up, including respiratory virus (RV), sexually transmitted infection (STI), gastrointestinal infection (GI), and urinary tract infection (UTI) assays. Seegene expects “3 Ct” technology to take syndromic testing to another level.
By detecting the causative pathogen, level of infection, and potential of co-infection, it will help determine the priority of treatment and enhance patient management. “3 Ct” technology also increases testing capacity. Such features are expected to improve the service and cost-structure of the medical sector once “3 Ct” technology is widely utilized.
Seegene’s first “3 Ct” technology applied product, Allplex HPV HR Detection, is designed to detect 14 high-risk human papillomavirus (HPV) types that can cause cervical cancer. It also provides the individual Ct value of each of the types allowing quantitative analysis regarding infection level. Early detection of HPV contributes to the prevention and management of cervical cancer. HPV products from other industry players provide individual Ct values for two high-risk types, HPV 16 and 18.
The Allplex HPV HR Detection is planned for launch within the first half of this year. The product will also be compatible with Seegene’s fully automated all-in-one system.
“HPV genotyping is essential for a good follow-up of a patient to observe the emergence, persistence or clearance of each genotype,” says HPV expert Sebastien Hantz, Professor at the Faculty of Medicine at the University of Limoges in France. “Seegene is a company very involved in the development of molecular diagnostics tests for the detection of different pathogens. For certain clinical situations, like respiratory infections, syndromic testing is very useful.”
Featured image: Seegene unveils world’s first commercialized ‘3 Ct’ PCR assay. Photo: Seegene