How MALDI-TOF MS is revolutionizing micro labs
By Gary Tufel
Matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI-TOF MS) provides microorganism identification results in minutes versus hours for phenotypic methods. Such rapid identification is achieved by detecting and analyzing the organism’s proteomic fingerprint.
And while MALDI-TOF MS is still a relatively new technology for microbiology labs, having really emerged only in the past decade, many believe that the technique has already brought about a revolution in the microbial identification of bacteria (including mycobacteria), yeast, and mycelia fungi. Robert Jerris, MD, medical director for microbiology at Children’s Healthcare of Atlanta, points to the Multiflex Biotyper by Bruker Daltonics Inc, Billerica, Mass, and the Vitek MS by bioMérieux, Durham, NC, as representative of both the present and future of microbial identification systems.
Steven Gygi, PhD, professor of cell biology and head of the Thermo Fisher Scientific Center for Multiplexed Proteomics at Harvard Medical School, says, “From my viewpoint, the most transformative technology in the field of proteomics is now available to apply to basic research questions. The advances are based around isobaric tagging techniques called tandem mass tags, which facilitate sample multiplexing.” With the help of groundbreaking and purpose-driven mass spectrometry instrumentation, he says, up to 10 samples can be compared on a proteome scale for protein expression or phosphorylation differences. Complex experimental designs that include time series or replicate conditions are possible because the 10 treatments are measured simultaneously.
“In a few years, this technology will almost certainly make its way to routine use in the clinic. If one wanted to measure a cohort of 500 patient biopsies, their protein levels could all be compared to each other and the analysis would happen 10 at a time,” says Gygi. “In truth, there are few limitations on moving from 10 to, say, 24 or 48 samples in a single experiment—so that protein measurements for even thousands of patients could be collected.”
The systems identify virtually any organism that can be grown on solid media, and they do so quickly and inexpensively, taking mere minutes for identification from the time the organism is placed in the MALDI-TOF MS system. “This is a giant leap forward from the phenotyping technology that is being replaced,” says Jerris. “This represents a big advance not only in speed and efficiency, but in cost savings, quality control, and a reduction in the hands-on time for techs performing the tests. It also reduces the number of specialized tests (phenotypic and genotypic) that used to take place in multiple subsections of the lab (mycology, mycobacteriology).
“MALDI-TOF MS replaces a myriad of phenotypic tests and reduces them to one test, one methodology for identification,” Jerris adds. “This is a big savings and a huge impact on patient care.”
Mass spec is mostly being used to diagnose infectious diseases, says Jerris, because of the particular need for early detection and the speed that mass spec offers. The use of MALDI-TOF MS to identify organisms to the genus and species level is becoming standard in many clinical microbiology laboratories. This data is provided to all clinicians, he says, and has played a big role in antibiotic stewardship, as empiric therapy may be initiated or a modification in therapy may take place simply by knowing the infecting organism(s).
EXPLOSIVE GROWTH
The growth of mass spec has been explosive, says Jerris, and it’s only just the beginning. “When FDA approves this process for all organism groups, the real expansion will begin.” Once that happens, he says that for medium-sized labs, initial investment in the technology will take 2 to 3 years to recoup, and for smaller labs, 3 to 5 years because the instruments are fairly costly. “But after that initial investment, it’s a no-brainer, because it’s standardized and inexpensive.”
Jerris says it’s important for labs to purchase service contracts, as instruments need maintenance and repair. Data drops are also necessary so that the manufacturers can perform remote diagnostics and tweak the systems when needed. “The savings will be really robust, but the most important element is the advantages the technology is bringing to patients,” he says.
“This is a revolution for microbiology on many levels, including workflow,” adds Jerris, “because there’s no longer a need for lengthy phenotype testing.” And the potential is only beginning to be fully appreciated. The biggest impediment, he says, is also a plus—that the testing is so accurate and advanced that it’s creating new issues.
“We are finding organisms that in the past we wouldn’t have known about. What do we do with an organism we’ve never heard of before? How do we do antimicrobic reporting and interpreting? We occasionally have to trace an organism back to older nomenclature and match it to standards for interpretation of susceptible, intermediate, or resistant,” says Jerris. “The Vitek MS is matched to the Vitek 2 antimicrobial susceptibility system (AST) and can accomplish this.
“But unlike bioMérieux Vitek, Bruker does not have an AST,” Jerris adds. “So Bruker has partnered for compatibility with the AST systems of other manufacturers—Beckman Coulter, Brea, Calif (MicroScan); Becton Dickinson, Franklin Lakes, NJ (Phoenix); and Thermo Fisher Scientific, Waltham, MA (Trek Aris), to cite a few—to effect a complete MALDI-TOF identification and antimicrobial susceptibility testing solution.”
FDA granted 510(k) de novo clearance to the bioMérieux Vitek clinical microbiology mass spectrometry system in August 2013.1 The system, which uses MALDI-TOF mass spec to identify disease-causing organisms, including bacteria and yeast, was the first such platform cleared for clinical use in the United States. Robin Patel, MD, director of the infectious diseases research laboratory at the Mayo Clinic, Rochester, Minn, called the agency’s clearance “a milestone for MALDI-TOF mass spectrometry for microbiology.”2 FDA clearance also provided the Vitek MS with a first-mover advantage—albeit a temporary one—as it battled Bruker’s competing Biotyper device for share of the US clinical microbiology market. Bruker’s system later received FDA clearance for identification of Gram-negative bacteria.
According to Bert Top, PhD, senior marketing manager for clinical diagnostics at bioMérieux, the Vitek MS is a mass spectrometer system using MALDI-TOF for the identification of microorganisms cultured from human specimens. The qualitative in vitro diagnostic device is cleared only for the diagnosis of infectious pathogens, but is indicated for use in conjunction with other clinical and laboratory findings to aid in the diagnosis of bacterial and yeast infections. Microbial specimens are analyzed based on the dynamics of particles projected by a laser shot in a vacuum tube, and an organism is identified. The resulting (protein) spectrum of mass distribution is interpreted according to a knowledge database developed by bioMérieux, Top says.
When FDA issued its clearance for the Vitek MS, Alberto Gutierrez, PhD, director of the Office of In Vitro Diagnostics and Radiological Health at FDA’s Center for Devices and Radiological Health, said, “The ability for laboratories to use one device to identify almost 200 different microorganisms is a significant advance in the timely identification of pathogenic microorganisms. Rapid identification of harmful microorganisms can improve the care of critically ill patients.”3
IDENTIFICATION IN 1 MINUTE
The Vitek MS can identify a microbial isolate in about 1 minute, while labor-intensive conventional test methods can take hours to weeks, says Top. Key to the clinical use of the system was the development of the innovative and proprietary population-based spectral database, in combination with data interpretation using a unique binning algorithm (patent pending). Top says that this technology breakthrough distinguishes the Vitek MS from other commercial MALDI-TOF MS systems, and provides:
- The largest FDA-cleared IVD database: consisting of 193 taxa, including Gram-positive bacteria, Gram-negative bacteria, and yeast. The species in the database account for approximately 95% of the bacteria or yeast pathogens regularly encountered in clinical laboratories. Another 562 less-common pathogens are also included in the database and can be reported with supplemental testing, as determined by the laboratory.
- High accuracy: 93.4%, with less than 1% of the isolates misidentified during clinical trials.
- High reproducibility: 99.7% correspondence with testing at three independent sites.
- Differentiation of closely related clinically important species: essential for diagnosis and treatment.
- Simple workflow: significantly reduces laboratory labor and reagent costs.
- Connectivity through bioMérieux’s proprietary Myla software: allowing microbial identification and antibiotic susceptibility data to be generated and reported to the healthcare provider in as little as 6 to 8 hours, a time frame that has been shown to have a major impact on reducing morbidity and mortality.
Top says the Vitek MS is rapidly changing the state of the art for large and small microbiology labs across the country?both in clinical and food safety testing. How the device is used varies from lab to lab; some save it for critical testing, while others use if for routine testing, he says. It requires very few supplies: just formic acid, matrix, and disposable slides.
“We are investigating the identification of other pathogens using Vitek MS, but the device is currently cleared for use in identifying a very broad array of pathogens responsible for many of the most dangerous infections known to humanity,” says Top.
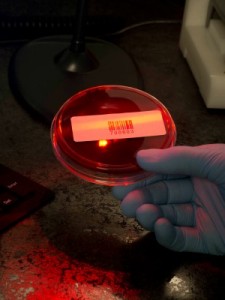
A technician scans unique barcoded identifiers from a cultured microplate, in preparation for testing on the Vitek mass spectrometer by bioMérieux.
Market response to the availability of the Vitek MS has been extremely positive, says Top, because the need for rapid diagnosis is vitally important right now. A recently published policy statement by the Infectious Diseases Society of America stated that, “The need for diagnostics that advance clinical care and public health has never been greater, and there is a critical window of opportunity to harness new technologies to address the greatest unmet needs.”4,5
“Our battle with antimicrobial-resistant organisms is a race against the clock,” says Top. “Today, most of the victims of these insidious infections are hospitalized patients who are recovering from cancer, organ transplantation, or other conditions that leave them immunocompromised. These are the most vulnerable patients, and as resistance spreads and our antibiotic armamentarium falters, drug-resistant pathogens will become a major public health threat to the healthy as well. The economic cost of antibiotic resistance in the United States, in 2008 dollars, has been estimated to be as high as $20 billion in excess healthcare costs, with additional costs to society as high as $35 billion a year for lost productivity,” he says.
Key to preventing the development of increased resistance and the spread of resistant pathogens is the rapid identification of the pathogen. “Only when clinicians know precisely what organism they are fighting can they empirically select the best antimicrobial drug for optimal therapy, thereby decreasing morbidity and mortality and fulfilling the goals of antibiotic stewardship,” Top adds. “If laboratories couple rapid identification with rapid antimicrobial susceptibility testing, we will be in a position to counteract the high degree of ‘drug-bug’ mismatch that exists today, primarily due to too little information, too late.”
REDUCING PATIENT STAYS, OVERALL COSTS
The Bruker MALDI Biotyper was used in a 2012 study at Houston Methodist Hospital that looked at antimicrobial stewardship programs to reduce hospital costs. The antibiotic stewardship program demonstrated reductions in patient length of stay and overall costs. The study showed an average length of stay was reduced by 2 days, and overall costs were reduced by almost $20,000 per patient.6
According to George Goedesky, Bruker vice president for MALDI Biotyper, Americas, and executive director for business development, Americas, more than 800 microbiology labs worldwide acquired the Bruker system prior to May 2013. But as of January 2015, that number has grown to more than 1,500. “Growth since the release of claims from FDA has met and exceeded our expectations,” Goedesky says. He attributes the system’s strong sales growth to its competitive cost, time to repair, and accuracy. Once implemented, the technology can reduce operational costs by 56% to 80%.
MALDI has changed the way labs are conducting microbiology testing, agrees Goedesky, but the revolution is far from over. Bruker is working on additional applications for the MALDI Biotyper, and has some research-use-only kits either in development or released. Already released is a specimen preparation kit called Sensityper that is used to identify bacteria directly from positive blood culture bottles, prior to growth of the organisms on culture media. This can result in identification of the organism days earlier than traditional biochemical-based methods that require recovery of the organism from the blood culture bottle. In development is a kit that can be used to detect bacterial resistance to some antibiotics. Identification is one valuable piece of data, as is the organism’s resistance patterns.
Michael H. Levi, PhD, director, and Wendy Szymczak, PhD, director’s assistant, in the clinical microbiology laboratory at Montefiore Medical Center, Bronx, NY, think that mass spectrometry will become the standard for identification of bacteria and fungi. “The quality of MALDI-TOF identification is better than phenotypic testing, and it improves efficiency and lowers costs. Despite what the initial cost might be, the technology is very user-friendly and cost-efficient; even smaller labs will find mass spec faster and easier to use.”
Levi says his lab selected the Bruker MALDI Biotyper because it was believed that Bruker had been in the field longer and was well established in Europe and Asia. In addition, the Montefiore microbiology laboratory has BD instrumentation and BD Epicenter, a lab-area network that enables the Bruker MALDI-TOF to interface with the current LIS. Montefiore also wanted to focus on the use of MALDI-TOF for identification of organisms from positive blood cultures using the Sensityper kit available from Bruker.
“Although use of the Sensityper kit slightly increases lab costs, valid identifications from blood culture bottles are available in 70% of bottles tested,” says Levi. “The ability to give the antibiotic steward team blood culture identifications within hours of Gram stain has proven to be an invaluable tool. This even extends to the ERs, where doctors are not used to seeing a final identification of a blood culture isolate. Overall, our experience at Montefiore with MALDI-TOF for blood cultures has been similar to that described by Dr Musser’s group at Methodist Hospital in Houston.”6
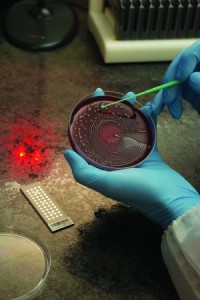
From a cultured microplate, a technician selects a colony for transfer to a slide, in preparation for testing using the Vitek mass spectrometer by bioMérieux.
“MALDI-TOF has replaced or will replace many phenotypic tests in the lab,” Levi adds, “and can be broadened to test for mycobacteria and filamentous fungi, thus giving the microbiology lab a universal identification platform.” By way of example, Levi recalls hearing that MALDI-TOF was to be used in testing for botulism toxins, replacing a mouse assay where a person had to observe a mouse after it was injected with a specimen thought to contain the toxin.
“We are planning to extend our current use to include MALDI-TOF for the detection of resistance mechanisms,” says Levi. “It’s a very powerful tool and state-of-the-science technology for the foreseeable future.”
Mass spec is used extensively in other parts of the laboratory for additional assays and analytes, says Goedesky. In terms of microbiology—but only for the infectious disease portion and only for MALDI-TOF—the Bruker MALDI Biotyper is used in the fields of clinical, veterinary, pharmaceutical, public health, and food testing, with the majority of installations being at clinical sites. The system’s broad success, says Goedesky, is due to its unique value proposition—highest per-hour throughput (~200 ID/hour), which meets the needs of high-volume sites, coupled with a compact, benchtop platform, which meets the requirements of smaller facilities.
All users, regardless of field or institutional size, get microbial identifications that are comparable to 16s sequencing but at a far lower cost and a far quicker time to result. The system accomplishes this with a simple sample prep that is comparable to performing a spot indole test. Bruker is developing additional applications, including identifications from positive blood cultures, resistance mechanism testing using MALDI, and subtyping. “Some of our clinical collaborators have already published results from studies conducted on these future applications,” says Goedesky.
To his knowledge, Goedesky says, in the field of microbiology, only vendors are developing additional applications for the platform. “However, customers are using the on-board software tools to develop and validate their own applications. One such example is an Australian customer who developed a method for detection of a vanB clone of Enterococci using the Bruker instrument.”
In support of collaborators’ projects, Goedesky says, Bruker offers an advanced MALDI Biotyper course for interested researchers, and collaborates extensively with them on new potential applications. Applications in the field of oncology have been developed, and researchers are investigating MALDI imaging for oncology applications, including margin excision and staging.7 Mass spectrometry is also widely used for toxicology and metabolic analytes.
In the field of infectious disease, Bruker has released a research use only (RUO) specimen prep kit called Sepsityper for identification from positive blood cultures. Collaborators worldwide have published using this approach and have shown that it can produce microbial identifications from non-charcoal-containing blood culture bottles significantly faster than phenotypic methods, and can do so at lower costs, Goedesky says.

A technician scans barcoded identifiers from a slide prepared for testing on the Vitek mass spectrometer by bioMérieux.
What is on the cutting edge of clinical applications for mass spec? Already, bacterial identifications are providing timely and valuable information for clinicians. Next along the edge is resistance mechanism detection, and Bruker is working on a number of approaches for detection of resistance mechanisms using MALDI-TOF, with interesting results generated to date worldwide. Bruker has intellectual property in this field as a result of its own development, and has executed a further agreement to expand its IP, says Goedesky.8
“Bruker’s first approach is a functional assay that detects the degradation products of an enzyme’s effect on an antibiotic,” says Goedesky. “Extended spectrum beta-lactamases pose a serious healthcare challenge, as there are hundreds of mechanisms by which bacteria can produce these enzymes. An RUO kit (STAR-BL) will be available in 2015, and Bruker is working with a number of collaborators in the United States,” he says.
“In addition,” he says, “Bruker Daltonics has published on other MALDI-TOF tools for the detection of nonenzymatic resistance mechanisms. Rapid bacterial identification coupled with resistance mechanism detection could provide further, useful information for the clinician, and Bruker is committed to developing additional applications and tools for our clinical partners.”
Gary Tufel is a contributing writer for CLP. For further information, contact CLP chief editor Steve Halasey via [email protected].
REFERENCES
1. bioMérieux receives FDA clearance for Vitek MS clinical microbiology system. GenomeWeb. August 22, 2013.
2. MALDI mass spec poised for further expansion into clinical microbiology market pending FDA approvals. GenomeWeb. July 19, 2013.
3. bioMérieux announces US FDA clearance for Vitek MS, a revolutionary technology which reduces microbial identification from days to minutes reinforcing medical value of diagnostics. [press release] (Marcy L’Etoile, France: bioMérieux, August 22, 2013); available at: http://www.biomerieux.com/en/biomerieux-announces-us-fda-clearance-vitekr-ms-revolutionary-technology-which-reduces-microbial. Accessed January 21, 2015.
4. Caliendo AM, Gilbert DN, Ginocchio CC, et al. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis. 2013; 57(suppl 3):S139-S170; doi: 10.1093/cid/cit578.
5. Better tests needed to improve patient care, public health. [press release] (Arlington, Va: Infectious Diseases Society of America, November 7, 2013); available at: http://www.idsociety.org/2013_IDSA_Diagnostics. Accessed January 21, 2015.
6. Rodak S. Study: antimicrobial stewardship, mass spec lower costs, length of stay. Becker’s Infectious Control and Clinical Quality. May 16, 2013. Available at: http://www.beckersasc.com/asc-quality-infection-control/study-antimicrobial-stewardship-mass-spec-lower-costs-length-of-stay.html. Accessed January 21, 2015.
7. Deep MALDI-TOF MS. [Web page] (Boulder, CO: Biodesix, 2015); available at: http://www.biodesix.com/technology/deep-maldi-tof-ms. Accessed January 21, 2015.
8. Bruker and Erasmus Medical Center sign an exclusive licensing agreement for adding rapid beta-lactamase testing capabilities to the MALDI Biotyper system. [press release] (Bremen, Germany: Bruker, January 1, 2013); available at: http://ir.bruker.com/investors/press-releases/press-release-details/2013/Bruker-and-Erasmus-Medical-Center-Sign-an-Exclusive-Licensing-Agreement-for-Adding-Rapid-Beta-Lactamase-Testing-Capabilities-to-the-MALDI-Biotyper-System/default.aspx. Accessed January 21, 2015.










