Chronic myelogenous leukemia (CML) accounts for 15% to 20% of all leukemia cases. Most CML cases are caused by a translocation between chromosomes 9 and 22 that creates an abnormal fusion gene, BCR-ABL1. In patients with CML, monitoring the concentration of BCR-ABL1 gene fusions predicts disease progression and tells physicians how well patients are responding to tyrosine kinase inhibitor (TKI) treatment.
Last year, FDA granted premarket notification (510(k)) clearance to Bio-Rad Laboratories, Hercules, Calif, for its QXDx BCR-ABL %IS kit, which employs the company’s droplet digital polymerase chain reaction (ddPCR) technology to quantify the BCR-ABL1 transcript in order to track the effectiveness of TKI therapy.
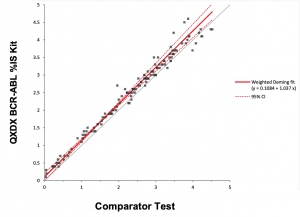
Figure 1. The QXDx BCR-ABL %IS assay demonstrated excellent correlation when compared to an existing FDA-cleared BCR-ABL test.
To determine how the newly cleared test performs in comparison to an existing FDA-cleared test, a team of researchers led by Nathan Sepulveda and Prasanthi Bhagavatula of Bio-Rad’s digital biology group undertook a comparative study of the tests. Results of the study, presented at the 2019 meeting of the Association for Molecular Pathology, demonstrated that the Bio-Rad test is reproducible across sites, instruments, and users, and offers excellent concordance with the previously cleared BCR-ABL test.1
The researchers also compared the QXDx BCR-ABL %IS test against current tests in the College of American Pathologists challenge—a neutral third-party assessment of the accuracy and reliability of clinical testing. The challenge compared the results of tests performed by the researchers on unknown samples to the results of tests performed by other users applying similar methods to determine whether the results aligned across methods.
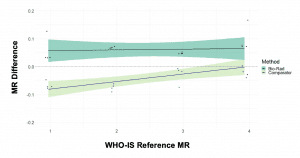
Figure 2. Both the QXDx BCR-ABL %IS assay and a comparator method showed close agreement when tested against the World Health Organization’s international standard.
The QXDx BCR-ABL %IS kit performed well in the challenge, closely matching the mean results for 10 different testing instances (r2 = 1.00). The kit also showed close agreement with both the existing FDA-cleared test (r2 = 0.99; Figure 1) and the World Health Organization’s international standard scale (Figure 2).
In addition, reproducibility was measured across multiple days, operators, sites, and kit lots. Day, operator, and site reproducibility were measured with 576 total measurements and 36 replicate tests for each sample. The measurements were taken from two replicates per run, for two runs per day, for three nonconsecutive days. Lot-to-lot reproducibility was measured across 108 samples from three lots. In terms of reproducibility, the kit showed negligible contributions to variance from day, operator, site, or kit lot.
“These results prove the kit’s real-world reproducibility and reliability compared to other tests on the market,” says Sepulveda.
For further information, visit Bio-Rad Laboratories.
Reference
- Sepulveda N, Bhagavatula P, Beppu L, Radich J, Shelton DN. Performance characteristics of the first FDA-cleared droplet digital PCR (ddPCR) IVD assay for monitoring chronic myelogenous leukemia. Poster presented at the annual meeting of the Association for Molecular Pathology, Baltimore, November 7–9, 2019. Available at: https://vertassets.blob.core.windows.net/download/d58cb6b5/d58cb6b5-cc6a-4f91-9f8d-21fec80a9cd7/bcr_abl_amp2019_10152019.pdf. Accessed February 25, 2019.