GenePOC Inc, Quebec City, PQ, Canada, a member of the Debiopharm Group, has announced that the company’s Carba assay for the detection of gene sequences associated with carbapenem nonsusceptibility has been awarded the CE mark.
The GenePOC Carba assay is a qualitative, in vitro diagnostic designed for the detection and differentiation of the blaIMP blaKPC, blaNDM, blaOXA-48-like, and blaVIM, gene sequences associated with carbapenem nonsusceptibility. The assay can provide results from one to eight samples in approximately 70 minutes using characterized carbapenem nonsusceptible isolated colonies of Acinetobacter baumannii, Enterobacteriaceae, or Pseudomonas aeruginosa.
The assay is performed on the company’s Revogene molecular diagnostic instrument, an automated and standalone device that interrogates single-use proprietary microfluidic cartridges with fluorescence-based real-time polymerase chain reaction technology to deliver an accurate diagnosis.
Carbapenemase-producing organisms (CPOs) are considered a serious global public health threat and are associated with significant morbidity, mortality, and hospital costs.1 In 2015, 13 of the 38 countries of the European Union and European Economic Area reported interregional spread or an endemic situation.2 High mortality rates, ranging from 30% to 75%, have been reported for patients with severe CPO infections.3
CPOs are adapted to spread in healthcare settings as well as in the community. To prevent transmission from CPO-positive patients, hospitals should consider enhanced infection control measures such as contact precautions, isolation, and dedicated nurses for patients who are confirmed CPO-positive.2
“The fight against carbapenemase-producing Enterobacteriaceae, a rising threat in healthcare facilities, requires rapid and efficient detection and differentiation,” says Thierry Naas, MD, professor of bacteriology services at Hôpital de Bicêtre.
“We are proud to receive the CE mark for our GenePOC Carba assay,” says Patrice Allibert, PhD, CEO of GenePOC. “A test which offers rapid and accurate results will contribute to the identification of colonized patients, therefore limit the spread of these organisms in healthcare settings and save on hospital costs. Our GenePOC Carba assay also demonstrates the power of our technology to be compatible with panel detection.”
For further information, visit GenePOC.
References
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol. 2009;30(10):972–976; doi: 10.1086/605922.
- Rapid risk assessment: carbapenem-resistant Enterobacteriaceae [online]. Stockholm: European Center for Disease Prevention and Control, 2016. Available at: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/carbapenem-resistant-enterobacteriaceae-risk-assessment-april-2016.pdf. Accessed June 2, 2019.
- Tischendorf J, de Avila RA, Safdar N. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: a systematic review. Am J Infect Control. 2016;44(5):539–543; doi: 10.1016/j.ajic.2015.12.005.
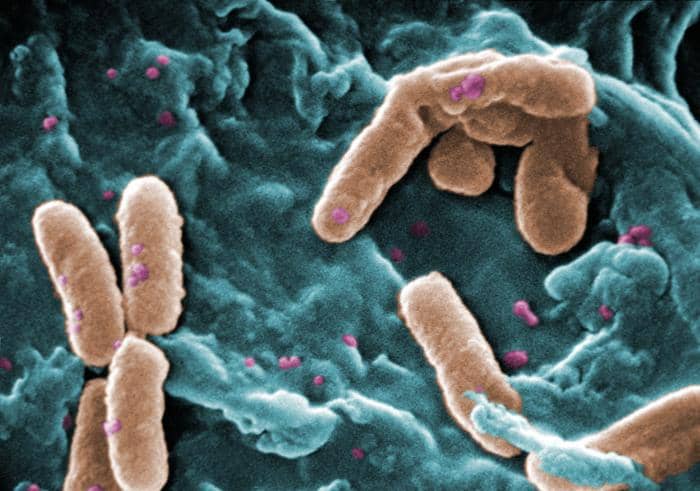
Featured image: A digitally-colorized scanning electron microscopic (SEM) image shows a number of rod-shaped, Gram-negative, Pseudomonas aeruginosa bacteria. Note the numerous, and significantly smaller, spheroid, purple-colored cocci bacteria also in the field of view. Photo by Janice Haney courtesy CDC Public Health Image Library (ID 10043).