The app integrates rapid diagnostic testing, artificial intelligence, and telehealth capabilities, while the company’s decentralized clinical trial platform aims to reduce study costs and improve data collection.
Biolabs International LLC has launched its Connected Diagnostics app and decentralized clinical trial platform, combining rapid diagnostic testing with artificial intelligence and telehealth services accessible via smartphone or tablet.
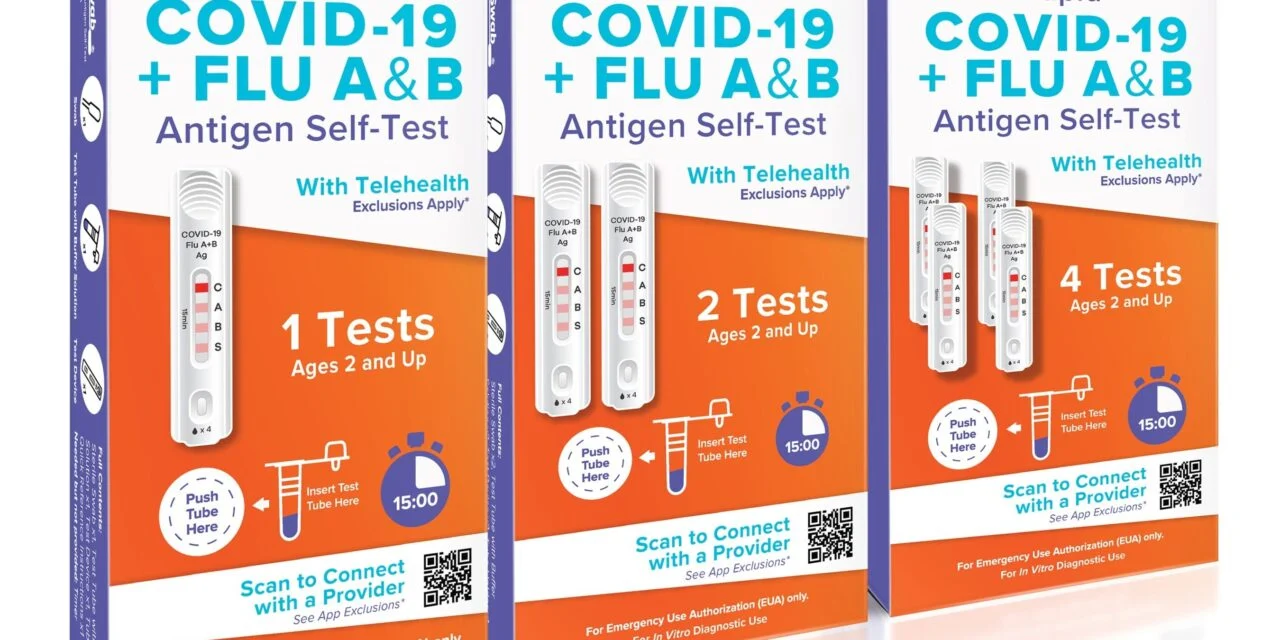
The San Diego-based healthcare innovation company’s platform allows consumers to self-administer SpeedySwab tests for COVID and influenza A and B at home, scan QR codes for instant app access, receive AI-interpreted results within minutes, and connect with healthcare professionals on-demand. The app is available on both Apple and Google app stores.
“Biolabs International is eliminating unnecessary doctor visits, reducing barriers to care, and setting new standards for accessible, AI-powered healthcare,” the company states in its announcement.
AI-Driven Result Interpretation
The Connected Diagnostics app, developed in collaboration with SafeHealth Systems and Mayo Clinic, uses proprietary algorithms to interpret lateral flow test results, designed to minimize human error and improve reliability. All data is encrypted and HIPAA-compliant, with secure sharing options for healthcare providers, employers, and public health agencies.
The platform integrates telehealth capabilities, enabling users to receive real-time medical advice and prescriptions based on their test results. The app includes step-by-step guidance and AI-driven result analysis, with options for immediate telehealth consultations when needed.
Decentralized Clinical Trial Platform
Beyond consumer testing, Biolabs’ decentralized clinical trial platform enables remote consent, test kit delivery, AI-based identity verification, and proctored testing sessions. The company says this approach reduces clinical study costs while aligning with FDA guidance on remote data collection.
The digitized trial process aims to improve participant retention, compliance, and accuracy while providing researchers with faster recruitment, automated data capture, and seamless integration with electronic medical records and regulatory reporting systems.
Retail Distribution and New Product Launch
SpeedySwab kits are now available nationwide at Walgreens, Kroger, and BevMo locations. Each kit is assembled in the US and packaged with QR-linked digital onboarding for quality control and rapid scale-up during public health emergencies.
The company also announced the launch of PowderTracer, a rapid 7-panel surface drug detection kit paired with an AI-assisted app. Designed for law enforcement, border control, corrections, and emergency responders, the system detects fentanyl, carfentanil, xylazine, methamphetamine, cocaine, K2, and THC in under five minutes.
The PowderTracer system automatically generates digital reports with photo documentation, removing subjectivity and ensuring consistent documentation for field testing applications.
Future Expansion Plans
Biolabs plans to expand its platform into women’s health, infectious disease, chronic condition management, and pet health applications. The company positions its approach as addressing healthcare accessibility challenges, particularly for individuals in rural, underserved, or high-risk environments.
The platform facilitates secure delivery of diagnostic results whether administered at home, in-clinic, or via kiosk directly to electronic health records, health information exchanges, and retail health ecosystems through the company’s SAFE platform.
Photo caption: SpeedySwab 3-in-1 COVID + Flu A & B test with telehealth
Photo credit: Biolabs International