By Eric Walk, MD, FCAP
Biomarkers and biomarker technology have been used to improve cancer diagnosis, aid in classification of biophysical properties, and provide prognosis and prediction of response to therapy. Now, laboratories are using biomarker technology to provide new clinical insights that can help improve cervical cancer screening and patient management. Recent studies have demonstrated that certain biomarkers can help identify women at high risk for cervical disease more accurately and efficiently, provide an effective triage strategy, and help guide clinical decisions about next steps for individualized patient care.
Biomarkers in Cancer
Biomarkers have played an important role in improving the evaluation and management of disease at different stages for decades. One of the best-known cancer biomarkers, prostate-specific antigen (PSA), was the first to receive FDA approval for the diagnosis and screening of prostate cancer.1 Since then, technological advances in molecular biology and high-throughput sequencing have dramatically expanded the contribution of biomarkers in cancer diagnosis and management.2,3
Nowhere is this scientific evolution more welcome than in cervical cancer screening, where the foe is one of the few cancers for which elimination is within reach.4 While tremendous advances have been made over the past few decades, first through the introduction of Pap cytology and more recently via high-risk human papillomavirus (HPV) testing, recent development of biomarker-based cytology addresses a continuing challenge in risk stratification by providing new and early insights about changes that are happening at the cellular level that might not be visible morphologically. The additional information from a biomarker test result may point to increased risk for disease that helps inform subsequent patient management decisions.
Molecular-based cervical cancer screening strategies may rely on HPV DNA tests alone or a combination of HPV and Pap cytology (co-testing) to obtain meaningful information about a woman’s risk for the presence of disease. Clinical guidelines often suggest a different patient management path when screening results are positive for one of the two highest-risk HPV genotypes, HPV 16 or HPV 18, compared to a positive result for the other high-risk HPV genotypes. Additionally, sometimes a co-testing scenario may give seemingly discrepant results, such as when a patient is positive for high-risk HPV yet has normal cytology.
The added insights from biomarker-based cytology are possible because of the nature of how HPV infections transform cervical tissue. HPV is detected in more than 99% of all cases of cervical cancer, pointing to this virus as the primary cause.5 While there are over 150 different types of HPV, only some pose high risk for cervical disease.6 Most women with an HPV infection will clear the virus naturally, but in a small number of women the virus can persist and, over time, develop into precancer or cancer. It is critical for clinical labs and healthcare providers to be able to differentiate women who are more likely to have a transient HPV infection from those who may have precancerous changes.
The Diagnostic Dilemma
While almost all cervical cancers are associated with 14 genotypes, HPV16 and HPV18 are the two highest risk types causing approximately 70% of cervical cancers and precancerous lesions.7,8 HPV infections are very common in the population, though not every HPV-positive woman will develop cervical cancer.9
Currently, Pap cytology and HPV genotyping information is used to categorize women into different risk levels. Cases with seemingly contradictory results are not uncommon and may lead to a management dilemma: surveillance or intervention, such as colposcopy? Both carry potential consequences. Clinicians can be concerned about losing patients asked to wait up to a year before retesting. Waiting may also result in missed diagnosis or disease progression. Although colposcopy is considered to be relatively safe, it may be unnecessary and this procedure may have a negative impact on patients’ psychological and reproductive health.10
The use of dual biomarkers offers a solution to the dilemma—without requiring more invasive procedures—because together they signal the presence of molecular alterations which show that HPV infections may have started their transforming activity of cervical tissues into precancerous lesions.
The Biology of Transforming HPV Infections
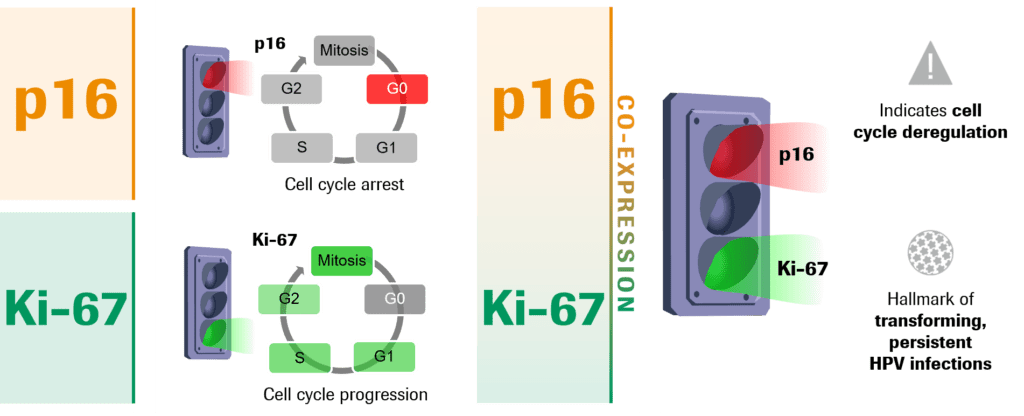
The simultaneous co-expression of the biomarkers p16 and Ki-67 within the same cell reveal molecular changes driven by HPV within cells of the cervix and indicate oncogenic activity is happening.
p16 is a tumor suppressor protein and is established as the only recommended biomarker to aid in the interpretation of cervical biopsy samples, in conjunction with H&E morphological interpretation. However, since cervical cytology samples lack the tissue structure context, and p16 can be occasionally expressed in metaplastic and endocervical cells, the utility of using only one biomarker in cytology samples is limited.11
Ki-67 is a well-known cell proliferation marker. In normal cells, p16 and Ki-67 are mutually exclusive. However, a transforming HPV infection deregulates the cell cycle, leading to the co-expression of both p16 and Ki-67 (Fig. 1). Detection of the two markers in the same cell indicates that the oncogenic transformation process driven by HPV has begun and is associated with a much higher likelihood for the presence of high-grade cervical disease.12
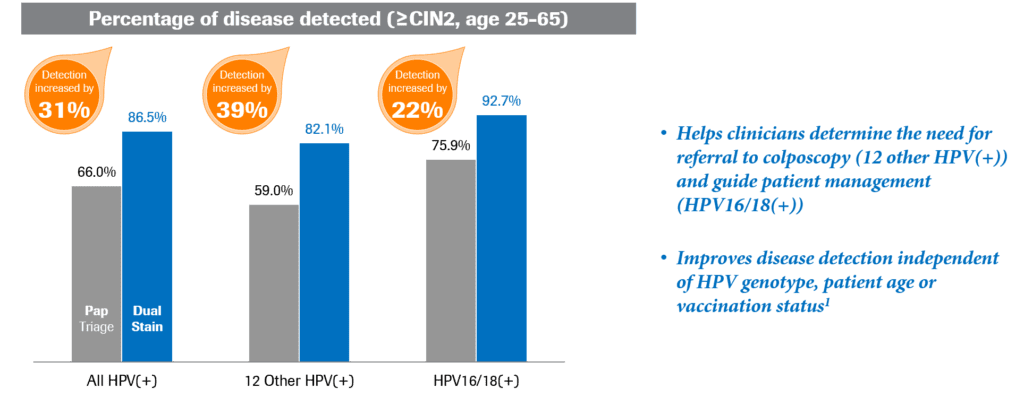
These two biomarkers provide an appropriate balance of sensitivity and specificity for detection of cervical precancer.13 Using biomarkers p16 and Ki-67 to look for changes on the cellular level in triage was shown to be significantly more sensitive than Pap cytology (86.5% vs. 66%; p<0.0001) when triaging HPV-positive women (Fig. 2).12
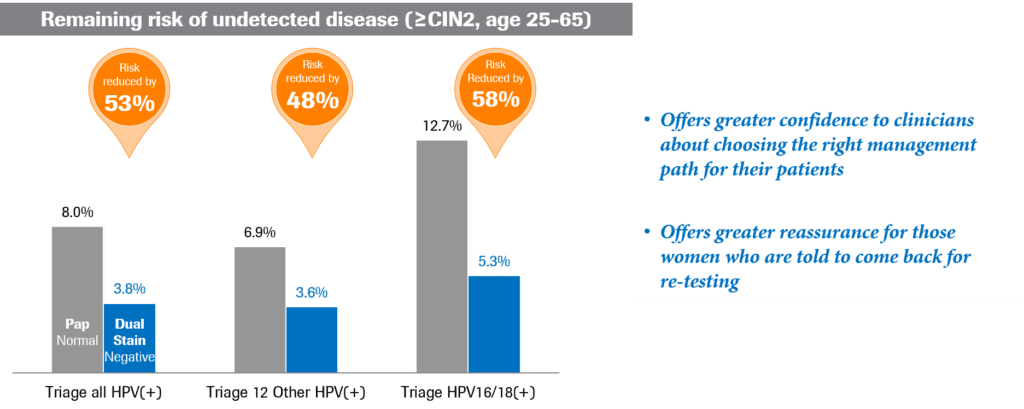
Additionally, use of these same markers can help cut the risk of undetected disease in half, particularly when comparing p16/Ki-67 dual-stained cytology with Pap cytology (Fig. 3).12
Multiple studies support the use of p16/Ki-67 dual-stained cytology for cervical screening, including the multicenter, prospective, registrational IMPACT (improving primary screening and colposcopy triage) trial.12,14 The IMPACT trial evaluated dual-stain biomarker technology as a triage test in common screening scenarios clinicians encounter that lead to different management decisions. The trial enrolled approximately 35,000 women, ages 25 to 65, who were undergoing routine cervical cancer screening across 32 clinical study sites (publication pending).
Based on data from the IMPACT trial, the FDA approved the first commercial dual-stain biomarker test for use in cervical cancer screening in March 2020 (CINtec PLUS Cytology, Roche Diagnostics).15 The test is performed on the same sample collected for a Pap liquid-based cytology test or HPV screening test, without requiring the patient to return to the clinician’s office.
Dual Staining and Risk Stratification
The application of biomarker technology to cervical cytology—immunocytochemical staining for proteins to characterize cells—plays a key role in cervical cancer screening because it provides objective, visually definitive evidence that fills gaps left by Pap cytology.
When used as a triage test for patients with positive high-risk HPV results, p16/Ki-67 dual staining provides greater sensitivity for precancer and improved risk stratification compared to traditional Pap cytology.16 Dual-biomarker staining is independent of cellular morphology and can more definitively identify patients who are at highest risk of disease and need immediate follow-up versus those whose infection may be more likely to self-resolve. Patients with a negative dual-stain result have low risk for disease, so allowing their body’s immune system more time to clear an infection may help reduce the potential for unnecessary treatment.17,18 Having access to these insights about whether HPV infections are actively transforming into cervical disease can provide clinicians with more definitive guidance for personalized patient management.
Closing the Gap in Cervical Cancer Prevention
The implementation of advanced biomarker technology in cervical cancer screening gives clinicians information about the molecular features of an HPV infection, to aid in clinical care decisions. The ability to know more about an individual woman’s infection and whether or not it is undergoing oncogenic transformation directly informs clinical decision-making and provides greater confidence to both clinicians and patients about the next steps in patient care.
Eric E. Walk, MD, FCAP, is chief medical and scientific officer at Roche Tissue Diagnostics in Tucson, Ariz. He is head of the department of medical and scientific affairs, overseeing medical affairs, clinical science, and pathology.Walk’s primary interests include personalized healthcare, translational oncology, and immunotherapy. He is a Phi Beta Kappa graduate of Johns Hopkins University and holds an MD from the University of Virginia Medical School. He is board certified in anatomic and clinical pathology and is a fellow of the College of American Pathologists. He currently is a member of the CAP Personalized Healthcare Committee.
Featured image: Cervical Cancer Screening Test write on a book and keyword isolated on Office Desk. Healthcare/Medical Concept (Photo 178127005 © bang oland | Dreamstime.com)
References
1. Pérez-Ibave DC, Burciaga-Flores CH, Elizondo-Riojas MÁ. Prostate-specific antigen (PSA) as a possible biomarker in non-prostatic cancer: A review. Cancer Epidemiol. 2018;54:48-55. doi: 10.1016/j.canep.2018.03.009.
2. Goossens N, Nakagawa S, Sun X, Hoshida Y. Cancer biomarker discovery and validation. Transl Cancer Res.2015;4(3):256-269. doi: 10.3978/j.issn.2218-676X.2015.06.04.
3. Vadas A, Bilodeau TJ, Oza C. The Evolution of Biomarker Use in Clinical Trials for Cancer Treatments.Washington, DC: Personalized Medicine Coalition/L.E.K. Consulting. Available at: http://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/The_Evolution_of_Biomarker_Use_in_Clinical_Trials_for_Cancer_Treatments.pdf. Accessed February 3, 2021.
4. Tsu VD, Ginsburg O. The investment case for cervical cancer elimination. Int J Gynecol Obstet. 138:(Suppl. 1): 69–73. doi: 10.1002/ijgo.12193.
5. Walboomers JM, Jacobs MV, Manos MM. et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12-9. doi:
10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F.
6. NIAID. Bioinformatics and Computational Biosciences Branch at the NIAID Office of Cyber Infrastructure and Computational Biology. Papillomavirus Episteme. 2016 Available at: https://pave.niaid.nih.gov/. Accessed February 10, 2021.
7. Li N, Franceschi S, Howell-Jones R, et al. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128(4):927-935. doi: 10.1002/ijc.25396.
8. de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8.
9. Holowaty P, Miller AB, Rohan T, To T. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst. 1999;91(3):252-258. doi:10.1093/jnci/91.3.252.
10. Fielding S, Rothnie K, Gray NM, et al. Psychosocial morbidity in women with abnormal cervical cytology managed by cytological surveillance or initial colposcopy; longitudinal analysis from the TOMBOLA randomized trial. Psychooncology. 2017;26(4):476-483. doi: 10.1002/pon.4163.
11. Darragh et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136(10):1266-1297. doi:10.5858/arpa.LGT200570
12. CINtec PLUS Cytology [package insert]. Indianapolis, IN: Roche Diagnostics Corporation; 2020.
13. Yu L, Fei L, Liu X, et al. Application of p16/Ki-67 dual-staining cytology in cervical cancers. J Cancer. 2019;10(12):2654-2660. doi: 10.7150/jca.32743.
14. IMPACT trial, Roche data on file.
15. Roche. Roche receives FDA approval for CINtec PLUS Cytology test to aid clinicians in improving cervical cancer prevention [press release]. March 11, 2020. Available at: https://www.roche.com/dam/jcr:99f1ae3b-7e36-41f6-a2fb-7e3af924f8da/en/roche-mediarelease-11032020-en.pdf. Accessed February 3, 2021.
16. Wright TC Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: Results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144(1):51-56. doi: 10.1016/j.ygyno.2016.10.031.
17. Wentzensen N, Clarke MA, Bremer R, et al. Clinical evaluation of human papillomavirus screening with p16/Ki-67 dual stain triage in a large organized cervical cancer screening program. JAMA Intern Med. 2019;179(7):881-888. doi: 10.1001/jamainternmed.2019.0306.
18. Clarke MA, Cheung LC, Castle PE, et al. Five-year risk of cervical precancer following p16/Ki-67 dual-stain triage of HPV-positive women. JAMA Oncol. 2019;5(2):181-186. doi: 10.1001/jamaoncol.2018.4270.





