Summary: BD has begun shipping its FDA-approved HPV self-collection kits to U.S. healthcare facilities, offering a less invasive and more accessible cervical cancer screening option that supports efforts to eradicate the disease.
Takeaways:
- BD’s self-collection HPV kits provide women with a private, less invasive alternative to traditional Pap smears, expanding access to cervical cancer screening.
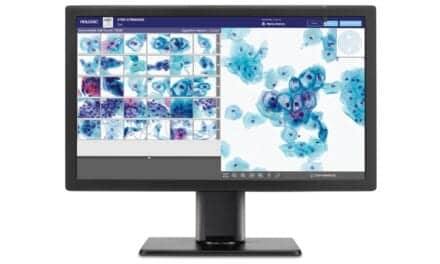
- The BD Onclarity HPV Assay offers extended genotyping, reporting six individual HPV strains to more precisely assess the risk of cervical precancer.
- BD is partnering with the National Cancer Institute in initiatives like the SHIP trial to evaluate the accuracy of self-collected HPV testing in diverse healthcare settings.
BD (Becton, Dickinson and Company), a leading global medical technology company, today announced the first shipments of its human papillomavirus (HPV) self-collection kits to health care facilities in the U.S., which supports national and global initiatives to eradicate cervical cancer.
This FDA-approved test provides a less invasive and more private option than traditional Pap smears for women. The test expands access to potentially life-saving cervical cancer screening to non-traditional settings like retail pharmacies and mobile clinics.
The FDA-Approved BD Onclarity HPV Assay
The BD Onclarity HPV Assay was recently FDA-approved for HPV primary testing without the need for a traditional Pap smear, which is performed with stirrups and a speculum. For a variety of reasons ranging from past experiences, or social or religious preferences, many women are not comfortable with the pelvic exam required for a traditional Pap smear. The availability of self-collection provides a less invasive testing option, potentially improving access to testing for individuals who face barriers to cervical cancer screening, including for those in geographic areas where there is not a clinician trained to perform cervical examinations.
Impact of Cervical Cancer
Cervical cancer is preventable, and screening plays a crucial role in early detection and prevention. According to the American Cancer Society, approximately 50% of cervical cancer diagnoses are in never-screened people, and 10% of diagnoses occur in under-screened individuals. In addition, 25% of women in the U.S. do not receive regular cervical cancer screening, according to the National Cancer Institute.
“I think women will be pleased to find that the steps for using the self-collection tests are easy to follow and that the test only requires a sample from the vaginal walls rather than the cervix,” says Jeff Andrews, MD, FRCSC, board-certified gynecologist and vice president of Global Medical Affairs for Diagnostic Solutions at BD. “Patients and providers can feel confident that the accuracy of self-collected vaginal samples for HPV testing is similar to clinician-collected cervical samples.[i]”
Further reading: HPV Self-Collection Is Recommended by Health Care Providers
BD Onclarity HPV assay is an HPV test that offers both extended genotyping and self-collection in a health care setting. HPV is the cause of virtually all cervical cancer, and HPV testing is the preferred screening method by the American Cancer Society in the United States. There are many strains (genotypes) of HPV viruses, with some strains posing a much higher risk for causing precancer and cancer than others. BD Onclarity reports six HPV strains individually, providing a more precise, accurate way to measure a women’s risk for developing cervical precancer by showing results for an extended set of individual HPV strains and enabling those strains to be tracked over time.
BD is working with the National Cancer Institute (NCI), part of the National Institutes of Health (NIH), in a public-private partnership called the Cervical Cancer “Last Mile” Initiative to address disparities in cervical cancer screening. As part of this initiative, BD is participating in the Self-collection of HPV testing to Improve Cervical Cancer Prevention (SHIP) trial to evaluate accuracy of Self-collection of HPV testing both in health care and other settings, including at home.
Photo: BD