 |
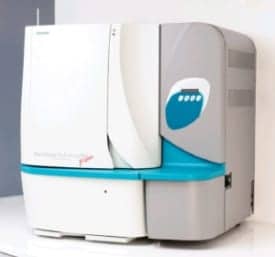
Geisinger Medical Center in Danville, Pa, a 403-bed health care system run by physicians, serves some 2.5 million people in 40 counties spanning 20,000 square miles in central and northeastern Pennsylvania. Its Janet Weis Children’s Hospital is the sole regional facility dedicated to children, focusing on specialties such as allergy, heart, cancer, hematology, neonatology, and rehabilitation. Paul Bourbeau, MD, who heads Geisinger’s microbiology lab, served on the Clinical and Laboratory Standards Institute committee that formulated the M40-A QC Standard for Microbiology Specimen Transport. Bourbeau’s lab was recently chosen by Copan Diagnostics Inc as the national launch site for its Walk-Away Specimen Processor (WASP).
The lab performs more than 400,000 tests per year, which includes in-house work and outreach for three nonaffiliated hospitals, prisons, nursing homes, physicians’ offices, and specialty microbiology testing for other labs. Some 40 FTEs shoulder the workload, but with the replacement pipeline all but drying up, Bourbeau’s biggest challenge is meeting the burgeoning demand for testing.
Volume has steadily risen in the 18 years since he first walked through the door, and Bourbeau is enthusiastic about validating a new instrument expected to pick up the slack in processing specimens—and freeing technicians for other tasks. The robotic WASP relies on a single, universal platform to automatically plant and streak specimens such as swabs, urine, and fecal samples, processing hourly some 180 urine samples, and throat or nasal swabs in most container styles.
“Microbiology historically has not had the benefit of the types of automation so common in chemistry or hematology labs,” Bourbeau says. Bourbeau earned his PhD in microbiology from Temple University Medical School and performed his postdoctoral training in microbiology in medicine and public heath. He is a Diplomate of the American Board of Microbiology. Bourbeau’s lab was a natural choice as a beta site, since the Geisinger scientist has actively studied specimen transport for more than a decade.
Bourbeau says he is impressed with the company’s engineering and history of high-quality innovation. He has maintained a decade-long relationship with the manufacturer, visiting its factory four times and helping to evaluate transport devices when they were first imported to the United States. The evaluations led to the M-40. Geisinger was chosen as the beta site for the first production version, and Bourbeau and his staff have spent the last month validating the system. “This is the first instrument trying to tackle processing across a wide specimen type. It is the only instrument like this in the world,” he says.
The WASP handles liquid-based specimens and selects streaking patterns—or users can devise their own, per the product’s stats. Bourbeau has not done so, in part because the software version he is using does not offer the option. The big-picture thinker is on it. “I was in Italy at the factory for about a week and worked with engineers to develop some streak patterns we thought would be useful for our lab and other users,” he says.
 |
| Geisinger Microbiology Lab Director, Paul Bourbeau, MD, validates the robotic instrument that plates and streaks samples. |
… Next
It could be another 3 months before the lab finishes validating all of the functions. The unit has not yet run through its paces at top speed, and technicians have not started working on specimens. Soon they will perform timing tests and wholesale comparisons with other methodologies. Nonetheless, Bourbeau says “everything we have done so far has met our expectations.
“The things we’re planning to do with this no one’s done on automation—plating stool specimens or respiratory specimens,” he says. “It may take us some time to figure out the best way to do this. The manufacturer can figure out ways to design the instrument, but it takes a facility to give real-world tests for the best modifications.”
While the WASP has the capability to plate a variety of specimens, laboratorians must first determine how to best process some types of specimens that have been manually plated in the past. For example, specimens—such as sputum, or endotracheal specimens—will need processing before being placed on the instrument. “We are in the process of devising how best to do this,” Bourbeau says, “appreciating that we will probably refine these protocols over time.”
Nasal screening for methicillin-resistant Staphylococcus aureus (MRSA) is among the pool of high-growth tests. Labs that are culturing for MRSA can accommodate an uptick in volume by placing specimens on the instrument, which can run around the clock. “It doesn’t take breaks, and will hold almost 400 pieces of media,” Bourbeau says. “You could load 50 specimens, walk away, and the people running the instrument are able to do other things. For large labs, the instrument can be on the front end.”
Bookmark This One
The unit includes a library of textbook-style streaking patterns and will offer customizing options, but, Bourbeau says, “by the time we’re done with our work, I don’t think there will be more options anybody will want. The library will satisfy most users.”
He plans to assemble the new cadre of users at next year’s meeting of the American Society for Microbiology to share their experiences. “We’re going to be doing true scientific studies—presenting, publishing, performing rigorous protocols for validating.”
Bourbeau’s schedule may be jam-packed, but he relishes the possibilities. “We are excited about the opportunity to participate in the first hospital-based, clinical evaluation,” he says. “We look forward to working with Copan, to improve even more operational utilization of the WASP. Because we are the first site, we have a lot of input into potential changes and innovations in the instrument.”
Looking further down the road, Bourbeau sees increasing consolidation of labs, particularly the labor-intensive microbiology labs, with small hospitals increasingly opting to outsource microbiology tests. “I am seeing growth in consolidated reference labs, and we will see growth in our demands for testing—and a substantial increase in the demand for molecular testing. The phenomenon is occurring throughout the country. The WASP will support that growth.”
Judy O’Rourke is associate editor of CLP.