 |
T. gondii Monoclonal Antibodies
Four different antigen specificities available
ViroStat, Portland, Me, has released a new set of Monoclonal antibodies to Toxoplasma gondii, which can affect certain immunocompromised groups. Four different antigen specificities are available, including SAG1 and MIC 3. All antibodies function in ELISA, IFA, and western blot immunoassay. These new antibody tools offer the potential for rapid antigen detection as well as by serology. A data sheet for the antibodies is available on the company’s Web site.
ViroStat
(207) 856-6620
www.virostat-inc.com
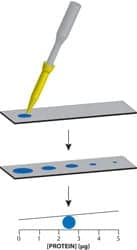 |
Protein dotMETRIC
For rapid estimation of protein concentration
The Protein dotMETRIC™ kit, from G-Biosciences, St Louis, is a 1-µL protein assay for the rapid estimation of protein concentration in cellular fractions, tissue, and cell lysates and chromatography purification fractions. The assay is compatible with sample-loading buffers and is ideal for confirming the amount of protein loaded on polyacrylamide gels. It is unaffected by the presence of common laboratory agents such as reducing agents, chelating agents, detergents, amines, sugars, chaotropes, and salts. Kits are supplied with an optional spot application device for increased reproducibility and test reliability. The spot application device allows application of samples using fixed-volume capillary tips and simplifies the task of applying the protein solution.
G-Biosciences
(314) 991-6034
www.gbiosciences.com
 |
Allergy Testing Systems
Comprehensive allergy testing portfolio
HYCOR, Garden Grove, Calif, offers a portfolio of allergy testing systems that feature a broad menu of allergens and a high level of quantitative accuracy. The Ultra-Sensitive EIA System is designed for moderate to higher-volume testing labs and combines quantitative accuracy, sensitivity, and fully automated robotics. HYTEC™ 288 Plus is a fully automated immunoassay system for the performance of allergy and autoimmune antibody testing, and for user-defined ELISA commercial or developed assays. Specifically designed to increase productivity and enhance efficiency in lower-volume labs, it is compatible with various interface protocols. The company’s allergy test portfolio can be run on both systems.
HYCOR
(800) 382-2527
www.hycorbiomedical.com
 |
DNA Extraction Kits
For use with the PapilloCheck HPV test
Greiner Bio-One, Frickenhausen, Germany, launches two variants of a specially adapted DNA extraction kit for its cervical (cervix uteri) cancer test system, PapilloCheck. By expanding the product portfolio for the test system, the company now offers a simple and rapid complete solution for detection of human papilloma viruses. The two DNA Extraction Kits, certified as CE-IVD (in vitro diagnostics) suitable for isolating human and viral DNA, are specially developed for extraction of the DNA from uterine cervix smears for subsequent analysis with PapilloCheck. Depending on the sample frequency or volume, two different extraction kit processing methods can be applied: either single-column processing using centrifugation or processing eight columns applying a vacuum system.
Greiner Bio-One GmbH
+49 (0) 7022 / 948-0
www.greinerbioone.com
 |
BD ProbeTec Amplified DNA Assays
Screen for chlamydia and gonorrhea
BD Diagnostics, Durham, NC, has received FDA clearance to utilize its BD ProbeTec™ Chlamydia trachomatis Qx and Neisseria gonorrhoeae QX amplified DNA assays, allowing labs to screen for chlamydia and gonorrhea using liquid-based cytology (LBC) samples collected during routine cervical cancer screening. Labs can then run the assays on the BD Viper™ system. The remaining specimen can be forwarded to the cytology lab for LBC slide preparation and testing.
BD Diagnostics
(201) 847-6800
www.bd.com



